Case Problem Answers Law for Business 19th Edition Chapter 15
[Previous page] [Table of Contents] [Next page]
Chapter 11 - Biological Safety Cabinets
Biological safety cabinets (BSCs) provide effective primary containment for work with infectious material or toxins when they are properly maintained and used in conjunction with good microbiological laboratory practices. The various classes and types of BSCs operate under the same basic principles. Personnel protection is provided through a continuous stream of inward air, known as inflow, which helps prevent aerosols from escaping through the front opening. The air that is exhausted into the surrounding containment zone or directly to the outside atmosphere is passed through high efficiency particulate air (HEPA) filters to protect the environment. Some classes of BSCs also offer product protection by using HEPA-filtered downflow to flush the cabinet interior of airborne contaminants and to prevent unfiltered inflow air from entering the work area. This chapter provides general descriptions of the different types and classes of BSCs. Different manufacturers may have unique design features and new technology in their BSCs. The physical containment requirements, operational practice requirements, and performance and verification testing requirements relating to BSCs in containment zones regulated by the Public Health Agency of Canada (PHAC) and the Canadian Food Inspection Agency (CFIA) are described in Matrices 3.7, 4.6, and 5.1 of the Canadian Biosafety Standard (CBS), 2nd Edition. Footnote 1
11.1 Classes and Descriptions
11.1.1 Class I
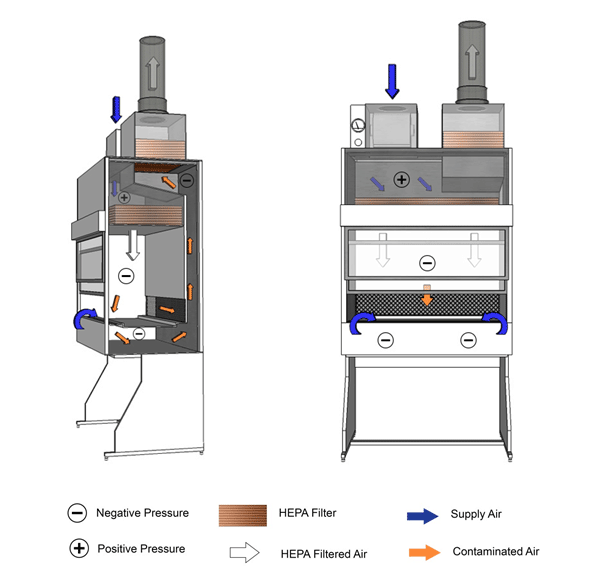
Class I BSCs provide personnel and environmental protection, but offer no product protection (Figures 11-1a and 11-1b). This type of cabinet is commonly used to enclose equipment (e.g., fermenters, homogenizers) or for procedures where product protection is not a concern (e.g., cage changing). Room air is drawn into the cabinet through the front opening, moves directly across the workspace, and is then discharged from the BSC through a HEPA filter. Class I BSCs can recirculate exhaust air into the containment zone, or exhaust directly to the outside atmosphere when hard-ducted to the facility's heating, ventilation, and air conditioning (HVAC) system. Since the air is never recirculated within the BSC, it is possible to work safely with minute quantities of volatile toxic chemicals if the BSC is hard-ducted. Class I BSCs are suitable for work with Risk Group 1 (RG1), Risk Group 2 (RG2), and Risk Group 3 (RG3) biological material. BSCs that are used as cage changing stations may require more frequent filter replacement due to filter loading.
11.1.2 Class II
Class II BSCs provide personnel and environmental protection; however, unlike Class I BSCs, they also offer product protection. Class II BSCs are further divided into four types: A1, A2, B1, and B2. Newer models do exist that can be configured as either a type A or a type B BSC during installation. The main differences between the types are the ratio of air exhausted from the BSC to the air that is recirculated within the BSC, and the type of exhaust system present. Some BSCs may recirculate air within the containment zone, while others may exhaust air directly to the outside atmosphere through dedicated ductwork. Class II Type A are the most commonly encountered BSC in a microbiology laboratory. RG1, RG2, and RG3 biological material can be handled safely in a Class II BSC. Risk Group 4 (RG4) biological material may be handled in a Class II BSC provided that a positive-pressure suit is worn. Table 11-1 summarizes the technical differences between the Class II cabinets.
11.1.2.1 Type A1
In this type of BSC, the room air and a portion of the BSC's recirculated air are drawn into the front grille and then HEPA filtered before flowing downwards over the work area (Figure 11-2). At approximately 6-18 cm above the work area, the downflow air splits, with approximately 50% of the air passing through the front grille and the other 50% passing through the rear grille, which then combine within a contaminated plenum. The contaminated plenum is either negatively pressured, or positively pressured and may be surrounded by negatively pressured plenums or ducts (Figure 11-2 illustrates a model with a positively pressured contaminated plenum). From this contaminated plenum, approximately 30% of the air passes through a HEPA filter before being exhausted out of the cabinet. The remaining 70% is recirculated and passed through a HEPA filter before flowing once again towards the work area. Type A1 BSCs can be exhausted into the containment zone or directly to the outside atmosphere through a thimble connection. Type A1 BSCs are never hard-ducted. Absolutely no work with volatile toxic chemicals or radionuclides is performed within this type of BSC as the recirculated air could cause a dangerous buildup of the toxic materials inside the BSC, or inside the containment zone.
11.1.2.2 Type A2
Type A2 cabinets are almost identical to type A1 cabinets; however, they have a greater inflow velocity and always have negatively pressured contaminated plenums or positively pressured contaminated ducts/plenums surrounded by negatively pressured ducts/plenums (Figure 11-3). In the event of a leak in the positively pressured ducts or plenums, this design feature draws air inward, thus preventing the contaminated air from escaping outward into the containment zone. This type of BSC is suitable for work with minute amounts of volatile toxic chemicals and radionuclides, if air is exhausted through a thimble connection.
11.1.2.3 Type B1
In this type of BSC, the room air and a portion of the BSC's recirculated air is drawn into the front grille and then directed through a HEPA filter located below the work surface (Figure 11-4). The air then flows upwards, through the side plenums and then through a second HEPA filter and downwards over the work area. Directly above the work surface and halfway between the front and rear grilles, the air splits and more than 50% of this contaminated air passes through the rear grille and through a HEPA filter before being exhausted out of the BSC directly to the outside atmosphere. The remaining air (less than 50%) passes through the front grille, mixes with the inflow air, and then passes through the HEPA filter located below the work surface. Type B1 BSCs are hard-ducted. Work with low levels of volatile toxic chemicals and trace amounts of radionuclides may be performed towards the rear of the work surface, where the air is discharged directly to the outside atmosphere.
11.1.2.4 Type B2
In this type of BSC, the supply blower draws room air into the top of the cabinet, through a HEPA filter, and then downwards over the work surface (Figure 11-5). The building exhaust system draws the air through the front and rear grilles into a contaminated plenum and then through a HEPA filter before being exhausted out of the cabinet directly to the outside atmosphere. Type B2 BSCs are hard-ducted. Work with volatile toxic chemicals and radionuclides may be performed in the BSC since the air is never recirculated within the BSC or within the containment zone. Reversal of airflow from the face of a BSC, also known as a puff-back, can occur in Class II type B2 BSCs, for example upon failure of the HVAC system, power, or the exhaust fan serving the BSC. Every effort is to be made to address puff-backs mechanically (CBS Matrix 3.7). When puff-backs occur in high containment zones, the laboratory is considered contaminated and full room decontamination may be necessary. Consideration should also be given to the amount of air required to operate this type of cabinet as it may lead to additional adjustments to balance the airflow in the containment zone.
| Type A1 | Type A2 | Type B1 | Type B2 | |
|---|---|---|---|---|
| Minimum average inflow velocity through front opening | 0.38 m/s [75 fpm] | 0.51 m/s [100 fpm] | 0.51 m/s [100 fpm] | 0.51 m/s [100 fpm] |
| Air patterns | 30% of the air is exhausted out of the BSC and 70% of the air is recirculated within the BSC | 30% of the air is exhausted out of the BSC and 70% of the air is recirculated within the BSC | >50% of the air is exhausted out of the BSC and <50% of the air is recirculated within the BSC | 100% of the air is exhausted out of the BSC |
| HEPA-filtered downflow air | Composed of mixed downflow and inflow from common plenum | Composed of mixed downflow and inflow from common plenum | Inflow air | Drawn from the containment zone or from the outside atmosphere |
| HEPA-filtered exhaust air | Recirculated to the containment zone or directly to the outside atmosphere | Recirculated to the containment zone or directly to the outside atmosphere | Exhausted through dedicated exhaust plenum to the outside atmosphere | Exhausted through dedicated exhaust plenum to the outside atmosphere |
| Type of exhaust | Can be thimble connected | Can be thimble connected | Hard-ducted | Hard-ducted |
| Contaminated ducts and plenums | Negatively pressured or surrounded by negatively pressured ducts or plenums; plenum may be positively pressured in some models | Negatively pressured or surrounded by negatively pressured ducts or plenums | Negatively pressured or surrounded by negatively pressured ducts or plenums | Negatively pressured or surrounded by negatively pressured ducts or plenums |
| Work with volatile toxic chemicals and radionuclides | No | Minute amounts if exhausted through thimble connection | Low levels of volatile toxic chemicals and trace amounts of radionuclides | Yes |
11.1.3 Class III
Class III BSCs provide product protection and maximum personnel and environmental protection (Figure 11-6). They are designed for work with RG4 pathogens and provide an alternative to the use of positive-pressure suits if the infectious material is exclusively handled within the Class III BSC. This type of BSC is completely enclosed; all penetrations are airtight and the BSC is kept under negative pressure (-200 Pa or lower, or as specified by the manufacturer) by a dedicated exhaust system. Manipulations are performed through attached heavy-duty long sleeved gloves that prevent direct contact with biological material. An inward directional airflow (IDA) of 0.7m/sec should be maintained when one glove is removed. The air from a Class III BSC is exhausted directly to the outside atmosphere through two consecutive HEPA filters or through a single HEPA filter followed by incineration. The introduction or removal of materials can be done in a variety of ways, including through a dunk tank, a double-door autoclave, a pass-through chamber that is decontaminated between uses, or a bag-in/bag-out system. Interlocks are used to prevent autoclave or pass-through chamber doors from being opened simultaneously (CBS Matrix 3.2). It is possible to join multiple Class III BSCs in a line to obtain a larger work area.
11.2 Installation of BSCs
Locating BSCs away from areas where airflow patterns may be disrupted (e.g., room air supply and exhaust grilles, doors, open windows, high traffic areas, and large pieces of equipment that generate heat) will help protect the fragile air curtain at the front of the cabinet (CBS Matrix 3.7). The following recommendations are to be considered with respect to the installation of BSCs:
- Consideration should be given to the use of bag-in/bag-out (or another procedure for the safe removal of filters) HEPA filters in situations where effective in situ decontamination is not feasible or possible. This allows for subsequent decontamination and disposal off-site (CBS Matrix 4.6).
- Adequate clearance should be provided between the exhaust outlet on top of the BSC and any overhead obstructions.
- Adequate clearance should be provided on each side of the BSC to allow access (Figure 11-7).
- BSCs should not be located directly opposite seated work stations, other BSCs, or chemical fume hoods. A reasonably safe distance, as determined by a local risk assessment (LRA), should be maintained to avoid operator collision.
- The thimble should be removable or designed to allow proper certification of the BSC (e.g., isolation damper to seal off the cabinet for decontamination, access port to allow scan testing of the HEPA filter).
- Hard-ducted BSCs should have exhaust blowers located at the terminal end of the ductwork. Exhaust flow failure(s) should signal an alarm to the user and activate an interlock system to prevent the cabinet blower from operating whenever the exhaust flow is insufficient (e.g., flow/electrical control) to prevent pressurization of the cabinet. Backdraft protection (i.e., damper) in the ductwork may be necessary to prevent reversal of airflow through the HEPA filter in the cabinet.
- Supporting BSCs with emergency power will help to maintain containment during emergency situations.
11.3 Testing and Certification
The required elements for testing and certification of BSCs are described in Matrix 5.1 of the CBS. Testing BSCs upon initial installation, annually, and after any repairs, modifications or relocation demonstrates that they are operating as designed. These activities can impact the integrity of the HEPA filters and plenums which could result in the release of infectious material and toxins. Most types of BSCs are tested in accordance with National Sanitation Foundation (NSF)/American National Standards Institute (ANSI) 49; however, for certain types (i.e., all Class I BSCs, Class II A1 BSCs, Class III BSCs, and custom BSCs), NSF/ANSI 49 is not applicable and the BSCs are tested in accordance with manufacturer specifications. Footnote 2 The following summarizes additional information to be considered for testing and certification of BSCs:
- On-site field testing should be performed by experienced and qualified individuals using test equipment with valid calibration certificates. The NSF accreditation program for BSC certifiers provides a list of individuals who have demonstrated their competencies by means of written and practical examinations.
- Interlocks (i.e., Class II Type B2 BSC internal cabinet supply fan and exhaust fan) should be tested in accordance with NSF/ANSI 49 to confirm that the internal supply fan shuts off whenever the exhaust air parameters fall outside of the setpoints.
- Alarms should be tested for detection of BSC or exhaust fan failure by simulation of alarm conditions.
- A label indicating the date of certification, the date when the cabinet is to be recertified, the standards or specifications to which the cabinet was tested, and the name of the certifier should be affixed to the cabinet exterior.
- During an exhaust fan failure, the time from the moment of alarm detection to the moment of airflow reversal from the face of the BSC (i.e., puff-back), if applicable, should be known for Class II B2 BSCs. If not conducted when installed, the cabinet alarm should be tested and adjusted to give the earliest possible warning to the user and to maximize the amount of time before the puff-back occurs.
- Positive pressure decay testing of Class III BSCs is done upon initial installation and when modifications have been made to the integrity of the cabinet, as per manufacturer's specifications. When modifications have not been made, annual integrity testing is done as well as any other tests recommended by the manufacturer. An example of an integrity test would be to smoke test the outside of the Class III BSC under normal operation. If no smoke is drawn into the cabinet from any of the seams, the integrity of the Class III BSC is acceptable.
Where a custom enclosure or the design of a BSC does not permit certification in accordance with NSF/ANSI 49, it is to be verified to meet the manufacturer's specifications, with minimum parameter values specified in Matrix 5.1 of the CBS.
11.4 Proper Use
Incorporating the elements outlined below into the applicable standard operating procedures (SOPs) to be followed by facility personnel is strongly recommended to encourage the proper and consistent use of a BSC by personnel to prevent exposures and the release of pathogens and toxins.
11.4.1 Start-Up Considerations
- Check that the sash is at the appropriate height. Adjust stool height so that the user's underarms are level with the bottom of the sash.
- Check the pressure gauges to verify that readings are within the acceptable range.
- If present, test the airflow alarm and ensure it is switched to the "on" position.
- Confirm inward airflow by holding a tissue at the middle of the edge of the sash to establish that it is drawn in.
- Disinfect the interior surfaces with a disinfectant effective against the infectious material and toxins used in the laboratory, allowing an appropriate contact time. If a corrosive disinfectant is used, the surface should be rinsed with water after disinfection.
- Assemble all materials required for manipulation and load into the BSC. Care should be taken not to overcrowd or block the front or rear grilles to prevent the appropriate airflow patterns from being compromised.
- When there is significant potential for splatter or splashes to occur during manipulations of infectious material or toxins, the work area should be lined with a plastic-backed absorbent pad.
- Place aerosol generating equipment (e.g., vortex mixer, sonicator) towards the back of the BSC, without blocking the rear grille.
- After loading material in the BSC, allow sufficient time for the air to purge and the airflow to stabilize before initiating work. This will be specified in the manufacturer's instructions, and is generally 3-5 minutes.
11.4.2 Working in the BSC
- Perform operations as far to the rear of the work area as reasonable. Ensure that elbows and arms do not rest on the grille or work surface.
- Avoid excessive movement of hands and arms through the front opening. Such movements disrupt the air curtain at the front of the BSC, which can allow contaminants to enter or escape the BSC. Arms should enter and exit the BSC slowly and perpendicular to the front opening.
- Keep a bottle of an appropriate disinfectant in the BSC while work is performed to avoid having to move hands outside of the BSC.
- Segregate non-contaminated ("clean") items from contaminated ("dirty") items. Work should always flow from "clean" to "dirty" areas (Figure 11-8).
- Material should be discarded in a waste container located towards the rear of the cabinet workspace. Do not discard materials in containers outside of the cabinet.
- Decontaminate the surface of all objects in the BSC in the event of a spill. The work area, including the inside surface of the window, should be decontaminated while the BSC remains in operation.
- Natural gas and propane should not be used in a BSC; sustained open flames (e.g., Bunsen burner) in BSCs are prohibited. On-demand open flames (e.g., touch-plate microburners) are to be avoided as they create turbulence in the BSC, disrupt airflow patterns, and can damage the HEPA filter (CBS Matrix 4.6). Non-flame alternatives (e.g., microincinerator, or sterile disposable inoculation loops) should be used whenever possible.
- Work in a BSC should only be conducted by one person at a time.
- Equipment creating air movement (e.g., vacuum pumps, centrifuges) may affect the integrity of the airflow and should not be used within the BSC.
- Windows that open should be kept closed when the BSC is in use.
11.4.3 Completion of Work in the BSC
- Upon completion of work, allow sufficient time for the air in the BSC to purge (i.e., pass through the filter) before disrupting the air curtain by removing hands or unloading material from the BSC. The purge time will vary by model and can be up to several minutes.
- Close or cover all containers.
- Surface decontaminate items before removing them from the BSC.
- Disinfect the interior surfaces of the BSC, including sides, back, lights, and interior of the glass, with a disinfectant effective against the pathogens in use, allowing an appropriate contact time (CBS Matrix 4.6). If a corrosive disinfectant is used, the surface should be rinsed with water after disinfection to avoid corrosion of the stainless steel surfaces.
- Routinely remove the work surface and disinfect the tray beneath it.
- Routinely wipe the surface of the lights within the BSC with a suitable cleaner or disinfectant (e.g., ethanol).
11.4.4 Ultraviolet Light Considerations
The use of ultraviolet (UV) germicidal lamps is strongly discouraged due to their limited effectiveness at disinfecting the inside of BSCs. Footnote 3Footnote 4 Personnel wishing to use UV irradiation in BSCs should receive training on the safe work practices required and the hazards of UV radiation beforehand, including the following elements:
- UV irradiation of the work area should only be used as a secondary method of disinfection in the cabinet. Never rely on UV irradiation alone to disinfect a contaminated work area.
- UV irradiation is ineffective if a microorganism is protected by dust, dirt, or organic matter. Footnote 4 A liquid chemical disinfectant should be the primary method of cleaning and disinfecting the interior of a BSC.
- UV irradiation does not penetrate into cracks or through the grilles of a BSC.
- UV irradiation can cause deterioration of various materials, including certain plastics and tubing.
- Never touch a UV bulb with bare hands as the natural oils from hands may leave a fingerprint and create dead space on the bulb's surface.
- UV bulbs should be cleaned frequently with an appropriate disinfectant.
- The UV lamp should be routinely tested with a UV meter to verify that the proper intensity (i.e., 40 µW/cm2) is being delivered at the appropriate wavelength (i.e., 254 nm) in the centre of the work area. Footnote 5
Figure 11-1a: Illustration of a Class I Biological Safety Cabinet (BSC)
Cabinet used in conjunction with building HVAC system. HEPA-filtered exhaust air is vented to the outside.
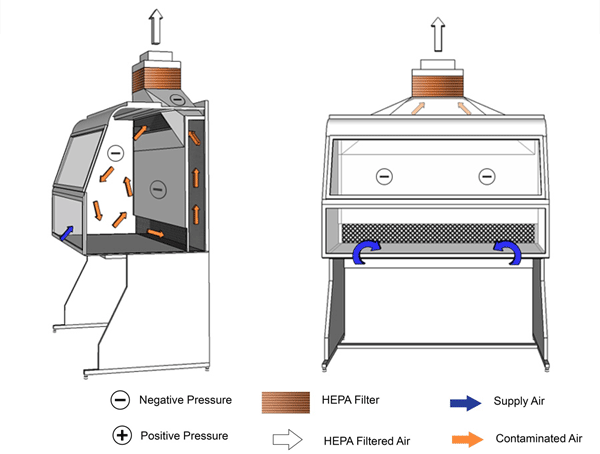
Text Equivalent - Figure 11-1a
In this figure, a Class one BSC is hard-ducted and functions using the building's HVAC system. Room air is drawn through the front opening of the cabinet and moves across the negatively-pressurized workspace. It is then drawn through an air grille situated at the rear of the cabinet, flows up a plenum and through a HEPA filter before being discharged to the outside environment.
Figure 11-1b: Illustration of a Class I Biological Safety Cabinet (BSC)
Cabinet shown is complete with internal motor/blower assembly. HEPA-filtered exhaust air is vented into the room.
Text Equivalent - Figure 11-1b
In this figure, a Class one BSC is shown with a motor and blower assembly. Room air is drawn through the front of the cabinet and moves across the negatively-pressurized workspace. It is then drawn through an air grille situated at the rear of the cabinet, flows up a plenum and through a HEPA filter before being discharged into the containment zone.
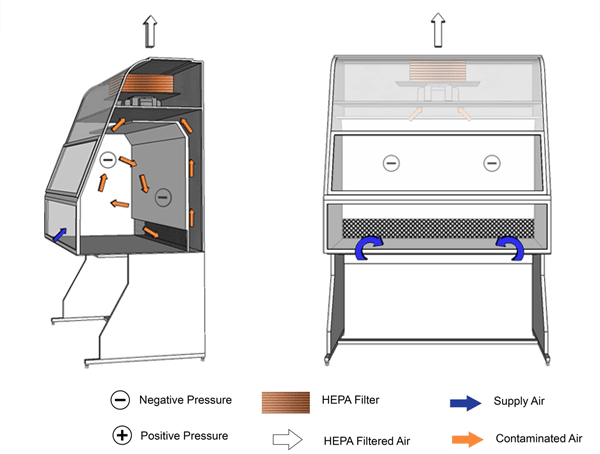
Figure 11-2: Illustration of a Class II Type A1 Biological Safety Cabinet (BSC) (with a Positively Pressured Contaminated Plenum)
Cabinet exhaust may be recirculated into the room or vented to the outside atmosphere through an air gap type (thimble) connection, as shown. Purple shading indicates positively pressured contaminated plenum.
Text Equivalent - Figure 11-2
In this figure, a Class two Type A-one BSC is shown with a thimble connection and a positively-pressured plenum. HEPA-filtered air from the top of the cabinet flows downwards towards the work surface. Above the work surface and halfway between the front and rear grilles, the filtered downflow air splits in two. One half of the downflow air passes through the front grille while the other half passes through the rear grille. Room air is also drawn into the front grille. The room air and downflow air are drawn through the grilles and into the negatively-pressured chamber underneath the work surface. The air is then drawn through the blower, pushed into the positively-pressured plenum and flows to the top of the cabinet. A portion of this air passes through the HEPA filter in the plenum before being recirculated towards the work area. The other portion passes through the HEPA filter located at the base of the thimble connection and is exhausted into the containment zone or to the outside atmosphere through the thimble connection. The Type A-one BSC shown contains a positively-pressured contaminated plenum.
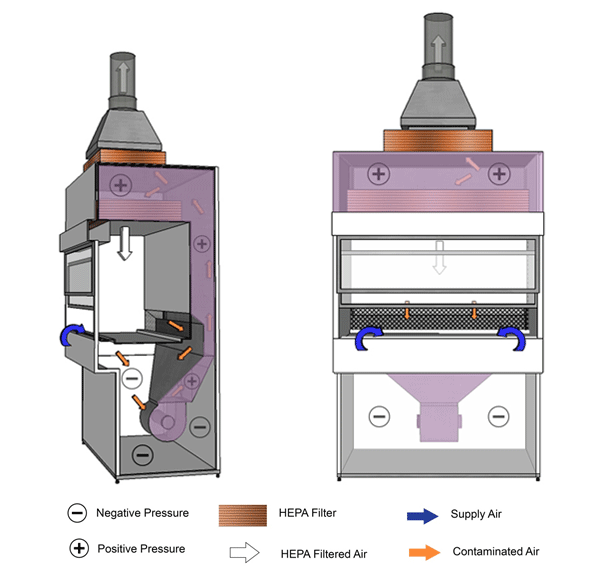
Figure 11-3: Illustration of a Class II Type A2 Biological Safety Cabinet (BSC)
Cabinet exhaust may be recirculated into the room or vented to the outside atmosphere through an air gap type (thimble) connection, as shown. Cabinet shown has a negatively pressured plenum.
Text Equivalent - Figure 11-3
In this figure, the BSC contains a thimble connection, and a positively-pressured contaminated plenum (between the blower and HEPA filters) surrounded by a negatively-pressured plenum. HEPA-filtered air from the top of the cabinet flows downwards towards the work surface. Above the work surface and halfway between the front and rear grilles, the HEPA-filtered downflow air splits in two. One half of the downflow air passes through the front grille while the other half passes through the rear grille. Room air is also drawn into the front grille. The room air and downflow air is drawn through the grilles and flows up the negatively-pressured plenum, through the blower, and into the positively-pressured plenum (between the blower and HEPA filters) at the top of the BSC. A portion of this air passes through the HEPA filter in the plenum before being recirculated towards the work area. The other portion passes through the HEPA filter located at the base of the thimble connection and is exhausted into the containment zone or directly to the outside atmosphere through the thimble connection.
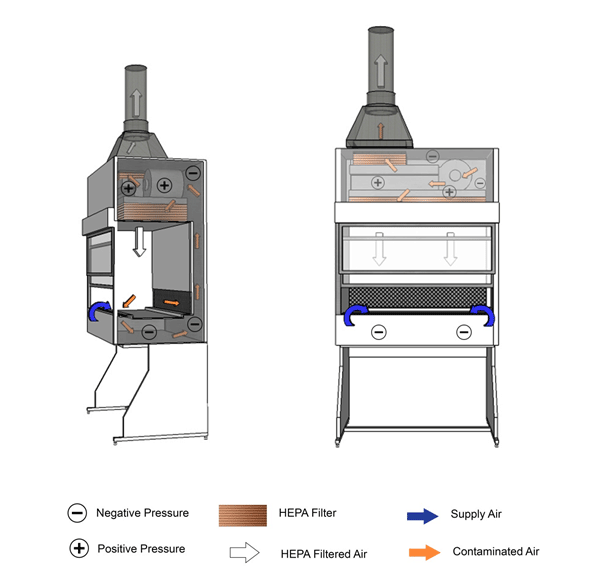
Figure 11-4: Illustration of a Class II Type B1 Biological Safety Cabinet (BSC)
Cabinet is vented to the outside atmosphere through a hard-ducted connection, as shown. The positively pressured plenum in this example is not contaminated, as the air is filtered before passing through the exhaust blowers.
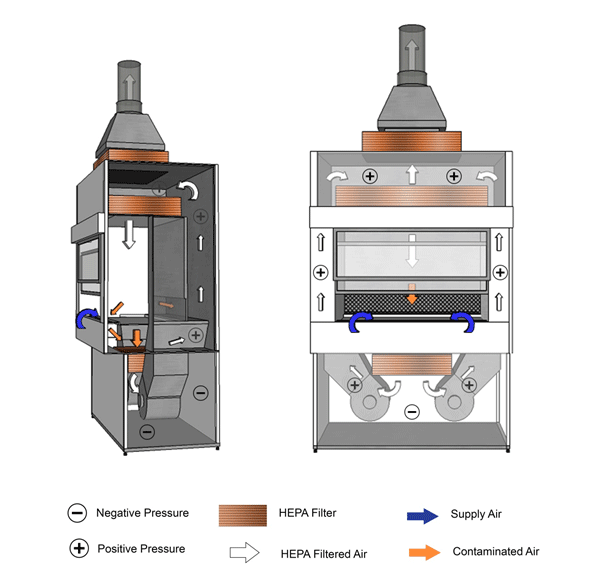
Text Equivalent - Figure 11-4
In this figure, a Class two Type B-one BSC is shown with a hard-ducted connection to the building's HVAC system. HEPA filtered air from the plenum flows downward and splits into two streams directly above the work surface, halfway between the front and rear grilles. The air drawn through the grilles is drawn through a HEPA filter by the blower and is pushed up thee plenums to the top of the BSC. A portion of HEPA filtered air flows downwards over the work area while the other portion flows through a HEPA filter before being exhausted out of the BSC to the outside atmosphere.
Figure 11-5: Illustration of a Class II Type B2 Biological Safety Cabinet (BSC)
Cabinet is vented to the outside atmosphere through a hard-ducted connection, as shown.
Text Equivalent - Figure 11-5
In this figure, a Class two Type B-two BSC is shown with a hard-ducted connection to the building's HVAC system. A supply blower pushes room air into the top of the cabinet. The contaminated air in the plenum remains physically separated from the room air from the supply blower. Room air is pushed into the top of the cabinet by the supply blower and is directed through the HEPA filter before being discharged downward into the cabinet work space. The downflow air splits into two streams directly above the work surface, halfway between the front and rear grilles. Room air is also drawn through the front grille before it can reach the work surface. The air directed through the grilles is drawn into the negatively-pressured plenum and flows to the top of the cabinet. It then passes through the HEPA filter and is directly vented to the outside atmosphere.
Figure 11-6: Illustration of a Class III Biological Safety Cabinet (BSC)
Cabinet is vented to the outside atmosphere through a hard-ducted connection, as shown.
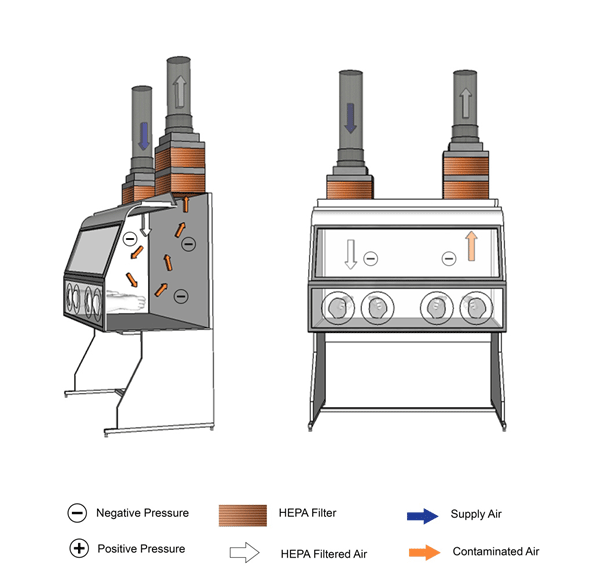
Text Equivalent - Figure 11-6
In this figure, a Class three BSC is shown with hard ducted supply and exhaust air. The BSC is completely enclosed; all penetrations are air-tight, and the BSC is kept under negative pressure. Manipulations are performed through attached heavy-duty long-sleeved gloves. Supply air is HEPA-filtered before entering the cabinet and circulates within the work space. The air from the work space is drawn into the exhaust duct and passes through two consecutive HEPA filters before being vented to the outside atmosphere.
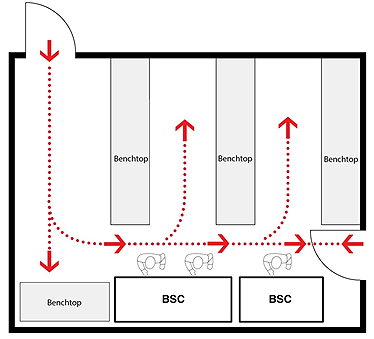
Figure 11-7: Representative Diagram Illustrating Location Considerations for Biological Safety Cabinets (BSCs)
(a) Well-located BSCs; minimum recommended clearances from a doorway and between BSCs when more than one BSC is installed in the room are shown. Specific BSCs may have different recommended clearances to prevent airflows from a neighbouring BSC from interfering with the protective air curtain. (b) Poorly-located BSCs; traffic, doorway, and neighbouring BSC are likely to disrupt the protective air curtain, and compromise personal, environmental, and product protections. Class II BSCs are designed and certified for use by a single individual.
(a) Well-located BSCs
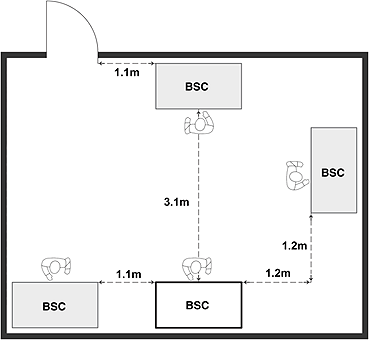
Text Equivalent - Figure 11-7a
This figure depicts two rooms in which BSCs are installed. In Figure 12-7(a), two BSCs are located along one wall, and two others along two of the other walls. These BSCs are well-located BSCs, respecting minimum recommended clearances from the doorway and between each of the other BSCs installed in the room. Specific BSCs may have different recommended clearances to prevent airflows from a neighbouring BSC from interfering with the protective air curtain. Figure 12-7(b) illustrates poorly-located BSCs in a different room layout. In this case, the two BSCs are located side by side along a wall, close to a door, and where traffic, the doorway, and the neighbouring BSC are likely to disrupt the protective air curtain, and compromise personal, environmental, and product protections. In addition, the figure shows two persons wording in one of the BSCs. Class II BSCs are designed and certified use by a single individual only.
(b) Poorly-located BSCs
Text Equivalent - Figure 11-7b
This figure depicts two rooms in which BSCs are installed. In Figure 12-7(a), two BSCs are located along one wall, and two others along two of the other walls. These BSCs are well-located BSCs, respecting minimum recommended clearances from the doorway and between each of the other BSCs installed in the room. Specific BSCs may have different recommended clearances to prevent airflows from a neighbouring BSC from interfering with the protective air curtain. Figure 12-7(b) illustrates poorly-located BSCs in a different room layout. In this case, the two BSCs are located side by side along a wall, close to a door, and where traffic, the doorway, and the neighbouring BSC are likely to disrupt the protective air curtain, and compromise personal, environmental, and product protections. In addition, the figure shows two persons wording in one of the BSCs. Class II BSCs are designed and certified use by a single individual only.
Figure 11-8: Representative Diagram of a Recommended Layout of Materials and Workflow inside a Biological Safety Cabinet (BSC)
The direction of workflow from "clean" (i.e., less contaminated) to "dirty" (i.e., higher contamination) is indicated.
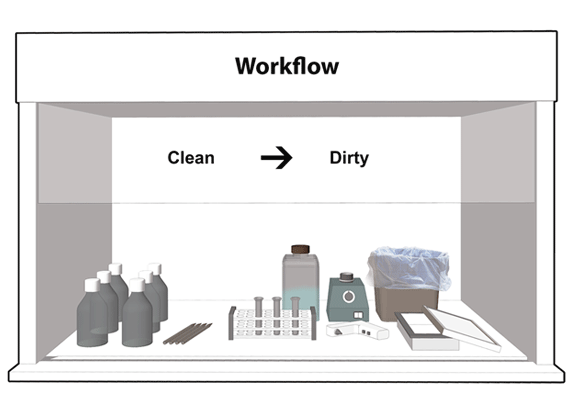
Text Equivalent - Figure 11-8
This figure shows a BSC set up for work, with clean reagents and pipets placed to the left, a rack with tubes in the middle, solid and liquid waste containers to the back and right, and a waste tray for pipets to the right. A vortex mixer is placed towards the back of the work area, and a cordless pipetting device near the centre, beside the rack. The direction of workflow goes from the "clean" side (i.e., less contaminated) to the "dirty" side (i.e., higher contamination).
References
- Footnote 1
- Government of Canada. (2015). Canadian Biosafety Standard (2nd ed.). Ottawa, ON, Canada: Government of Canada.
- Footnote 2
- NSF/ANSI 49-2014, Biosafety Cabinetry: Design, Construction, Performance, and Field Certification. (2014). Ann Arbor, MI, USA: National Sanitation Foundation / American National Standards Institute.
- Footnote 3
- Burgener J. (2006). Position Paper on the Use of Ultraviolet Lights in Biological Safety Cabinets. Applied Biosafety: Journal of the American Biological Safety Association. 11(4):227-230, Retrieved 11/03, 2015 from http://www.absa.org/abj/abj/061104burgener.pdf
- Footnote 4
- Lawrence Berkeley National Laboratory. (2010) . Biosafety Manual - Appendix F: Decontamination and Antimicrobials. Retrieved 11/03, 2015 from http://www2.lbl.gov/ehs/pub3000/CH26/CH26_Appx_F.html
- Footnote 5
- United States Department of Health and Human Services, United States Centers for Disease Control and Prevention & United States National Institutes of Health (2009). Primary Containment for Biohazards: Selection, Installation and Use of Biological Safety Cabinets (2nd ed). In Richmond, J. Y., & McKinney, R. W. (Eds). Biosafety in Microbiological and Biomedical Laboratories (5th ed.). Washington DC, USA: United States Government Printing Office.
Chapter 12 - Safety Considerations for Equipment Used for Biological Work
In both laboratory work areas and animal containment zones, a wide variety of equipment can be used when handling infectious material or toxins. Equipment that is operated and maintained properly minimizes the risk of exposure and prevents the release of pathogens and toxins into the environment. An equipment maintenance program facilitates tracking and scheduling of inspections and repairs, and is an important component of a facility's Biosafety Manual. In addition, training of personnel on standard operating procedures (SOPs) pertaining to all containment zone equipment, such as centrifuges, microtomes, pipetting aids, vacuum systems, and pass-through chambers is critical to the provision of a safe work environment. This chapter provides guidance on the safe use of select equipment used in laboratory work areas and animal work areas for activities with biological material. Local risk assessments (LRAs) are conducted to identify risks, examine procedures, and develop safe work practices for all equipment that, in turn, can be developed into SOPs. The minimum biosafety requirements for the equipment described in this chapter are specified in Chapters 3, 4, and 5 of the Canadian Biosafety Standard (CBS), 2nd Edition; the specific matrices referencing the equipment type being described are provided in each of the following sections. Footnote 1
12.1 Centrifuges
There is a risk of infectious aerosol generation when a centrifuge is used (e.g., tube breakage, improper use of safety cups or rotors, or lack of proper maintenance). The following points highlight some requirements and recommendations for centrifuge use when working with infectious material or toxins:
- The outside surface of cups and rotors should be decontaminated, as required.
- Equipment should be used in accordance with the manufacturer's instructions, which includes the balancing of rotors to prevent rotor damage or explosion.
- Plastic tubes that are suitable for centrifugation should be used (e.g., thick wall external thread plastic tubes with screw caps).
- Sealed centrifuge cups or rotors are to be used to prevent the release of aerosols during centrifugation, and the integrity of the cup or rotor seal regularly inspected (CBS Matrix 4.6).
- Cups and rotors with samples of infectious material or toxins are to be unloaded inside a biological safety cabinet (BSC) to protect against the release of infectious aerosols or aerosolized toxins (CBS Matrix 4.6).
- Sufficient time for aerosols to settle should be allowed prior to opening cups and rotors.
- The use of centrifuges inside a Class II BSC will disrupt the airflows and compromise the protection provided by the BSC, and should be avoided.
12.2 Microtomes
Microtome work with infectious material or toxins that may not have been inactivated by fixation should be performed in a low traffic dedicated area (e.g., taped off) to prevent tracking of wax shavings within or out of the containment zone. Care should be taken as the floors in histopathology areas tend to be quite slippery from the wax. Disposable shoe covers, dedicated to this area, should be worn; slip-resistant shoe covers are recommended for such areas. Respiratory protection should also be worn if deemed necessary by an LRA. Troughs may be installed on the edge of the work bench to contain excess shavings. Care should be taken when installing or removing microtome blades; non-disposable blades can be cleaned with an instrument, rather than by hand, to prevent contact with the blade. When manipulating tissue potentially infected with pathogens or prions, additional personal protective equipment (PPE) such as cut-resistant gloves can be worn to reduce the risk of exposure or injury.
12.3 Blenders, Sonicators, Homogenizers, Shaking Incubators, and Mixers
The operation of blenders, sonicators, homogenizers, mixers, shaking incubators, and other similar equipment can generate aerosols. The following points highlight some requirements and recommendations when using these types of equipment:
- Laboratory equipment and associated accessories specially-designed to contain infectious aerosols can be used for manipulations of pathogens and toxins. For example, cup horn sonicators allow sonication of samples within a contained vessel without direct contact with the material being processed.
- When equipment designed to contain infectious aerosols is not available, equipment should be operated in a BSC (only if the equipment does not disrupt airflow patterns) (CBS Matrix 4.6) or another primary containment device.
- Time for aerosols to settle should be allowed before opening or removing the covers.
12.4 Bunsen Burners
Bunsen burners are commonly used for heating (e.g., fixing cells onto slides) and sterilization (e.g., inoculation loops). Aerosolization of infectious material can occur when inoculation loops are sterilized in the open flame of a Bunsen burner; microincinerators or disposable loops are recommended as alternatives. Sustained open flames are prohibited from use inside a BSC as they will disrupt the airflow patterns, decrease the user protection provided by the air curtain, and have the potential to damage the filters (CBS Matrix 4.6). When suitable non-flame alternatives are not available, touch-plate microburners that provide a flame on demand may be used. The correct operation of BSCs is discussed in Chapter 11.
12.5 Microincinerators
Microincinerators can be used as an alternative to Bunsen burners, especially for use in a BSC. They are often equipped with shields to minimize the dispersal of infectious aerosols. When used in a BSC, the microincinerator should be placed at the rear of the working area inside the cabinet to help minimize disruption of the air curtain at the front of the cabinet.
12.6 Disposable Loops
Single-use disposable loops are sterile, and can be used in a BSC as an alternative to reusable loops that require sterilization with a burner or microincinerator; however, they will add to the amount of waste requiring decontamination. Disposable loops should be placed in a leak-proof, puncture-resistant waste container immediately after use.
12.7 Pipetting Aids
Pipetting aids minimize the risk of aerosol generation when used properly; they also eliminate the risk of ingestion of infectious material through oral pipetting, which is prohibited at all containment levels (CBS Matrix 4.6). Discharging liquid from a pipette and the aspirate/expel action used to mix cultures can create aerosols. The following points highlight some requirements and recommendations for the safe use of pipetting aids:
- use a BSC when pipetting infectious material or toxins (CBS Matrix 4.6);
- work over plastic-backed absorbent material; the droplets will be absorbed rather than "splash";
- use pipettes calibrated "to deliver", which reduces the risk of creating aerosols by retaining the last drop in the tip;
- use plastic pipettes instead of glass pipettes whenever possible;
- use filtered serological pipettes with pipette aids and filtered pipette tips with micropipettors, as these will prevent contamination of the pipetting device;
- use appropriate decontamination procedures for pipette aids and micropipettors when non-filtered tips are used or when the pore size of the pipette filter is insufficient for filtering the pathogen(s) or toxin(s) in use;
- don't mix liquids by bubbling air from a pipette through the fluid or by alternate suction and forceful expulsion through the pipette;
- discharge liquids as close as possible to the wall of the tubes or to the surface of media;
- avoid forcefully aspirating or expelling liquids from the pipette;
- pipet tips can be ejected directly into a container (e.g., bottle, beaker) for subsequent decontamination or bag for autoclaving; and,
- pipettes should be decontaminated with a suitable disinfectant immediately after use;
- serological pipettes can be laid horizontally in a pan and completely immersed in a disinfectant (care should be taken when moving the pan to avoid a spill hazard); or
- serological pipettes can be filled with disinfectant and left to drain by gravity into an oversized waxed cup in an autoclave bag (the bag can be closed over the pipettes and this can be autoclaved as a whole in an upright position before reuse).
12.8 Vacuum Pumps and Systems
Vacuum systems are used to create a void in filtration units and to aspirate liquids. The most common laboratory vacuum systems are centralized vacuum (void) systems, vacuum pumps, or a faucet aspirator vacuum pump attached to the water supply. The primary concern with vacuum pumps is that the process of aspiration can cause the aerosolization of infectious material or toxins, and subsequent contamination of the vacuum line and pump or system. A device (e.g., in-line high efficiency particulate air [HEPA] filter or 0.2 µm filter with disinfectant traps) is used to protect the vacuum system from internal contamination (CBS Matrix 3.7). This is visually demonstrated in Figure 12-1. A maintenance program for the regular inspection and replacement of in-line filters (CBS Matrix 4.6) will help prevent a breach in filter integrity and containment. For high containment zones, the use of portable vacuum systems instead of centralized vacuum systems will minimize the risk of a containment breach. Decontamination methods and selection of chemical disinfectants are discussed in Chapter 15.
12.9 Chemical Fume Hoods
Chemical fume hoods are designed for the manipulation of chemical substances, particularly volatile substances. Materials exhausted from chemical fume hoods are filtered with recirculation of the remaining air stream, or exhausted directly to the outside atmosphere. If required, filters are selected according to the type of contaminant to be removed, the efficiency required to meet occupational and environmental exposure limits, and the required residence time. Locating filters upstream of the exhaust fan, and in such a way as to allow replacement without contaminating the surrounding area, keeps contaminated ducts under negative pressure and prevents the release of chemical substances. Testing and replacement should be more frequent for filters used to trap chemicals that are capable of degrading the filter. It is the responsibility of the facility to determine the compatibility of specific chemicals with various filters, and to determine the appropriate replacement frequency. The inclusion of exhaust air treatment devices (e.g., activated carbon filters) are to be consistent with applicable local regulations.
Chemical fume hoods are not designed for the manipulation of infectious material or toxins, and consideration should be given to minimizing the placement of chemical fume hoods in high containment zones; instead, Class II B2 BSCs, which are designed to handle infectious material or toxins as well as volatile chemicals and radionuclides, should be considered. Fume hoods that are located in high containment zones are to comply with the requirements for HEPA filtration of exhaust (CBS Matrix 3.5). Chemical fume hoods should not be located directly opposite or in close proximity to BSCs in order to prevent disruption of the protective air curtain. The installation of a HEPA filter upstream of the charcoal filter is recommended as a measure to protect the charcoal filter from contamination with infectious material and toxins. Class II B2 BSCs are further discussed in Chapter 11.
12.10 Pass-Through Chambers
Pass-through chambers allow for the safe movement of materials into and out of containment zones and are available in a variety of sizes and configurations, including wall-mounted types, floor-mounted types with built-in ramps, as well as the type integrated into a Class III BSC. Various methods of decontamination are available for different types of pass-through chambers, including moist-heat (autoclave), dry-heat (hot-box), and gas or vapour (fumigation). The choice of decontamination method will depend upon the nature of the items requiring sterilization, as well as the type of infectious material or toxins in use. Additional features, such as HEPA-filtered pass-through chambers, are also available. To prevent pass-through chamber doors from being opened simultaneously, they are usually equipped with door interlocks or visual and audible alarms (CBS Matrix 3.2). Decontamination methods and selection of chemical disinfectants are discussed in Chapter 15.
12.11 Cell Sorters
Cell sorters are used to physically separate a defined subpopulation of cells from a heterogeneous cell population. Footnote 2Footnote 3The risk associated with cell sorters can be attributed to both the nature of the sample (i.e., the presence and type of the infectious material or toxins in the sample), and to the equipment itself (e.g., the use of droplet-based cell sorting, which uses jet-in-air technology that can produce aerosolized droplets). Droplet-based cell sorting involves the injection of a liquid stream carrying the cells through a narrow nozzle that vibrates at a high frequency. High-speed cell sorters with jet-in-air technology use even higher pressures and nozzle vibration frequencies, and consequently produce a large amount of aerosolized material. An LRA can be conducted to determine the physical containment and operational practices necessary to safely work with infectious material or toxins in a cell sorter. A cell sorter may need to be housed inside a ventilated enclosure that is custom-built by the same manufacturer for use with pathogens and toxins if it is not able to be housed inside a BSC. Custom ventilated enclosures are required to be certified to the manufacturer's specifications and demonstrate integrity as described in Matrix 5.1 of the CBS.
12.12 Compressed Gas Cylinders
Compressed gas cylinders can leak, and difficulties can be encountered when maintaining, replacing, and decontaminating tanks. In containment level 4 (CL4) zones, there is the added concern that positive-pressure suits could be damaged when changing tanks or fitting regulators. For these reasons, it is recommended that compressed gas cylinders be located outside high containment and prion zones where possible. Fire extinguishers and backup air cylinders may be required in high containment zones for personnel protection in life-threatening emergencies. Some containment level 3 (CL3) zones using sophisticated equipment (e.g., mass spectrophotometer, high performance liquid chromatography) may necessitate the use of small cylinders of reference gases in the containment zone that would be impractical to pipe in from outside the zone.
12.13 Additional Equipment Considerations for Prions
The following are additional equipment considerations for containment zones dedicated to prion work:
- dedicated laboratory work areas and equipment should be used, where possible;
- disposable equipment and laboratory supplies should be used when handling material known to contain prions;
- blunt cannulas can be used in place of needles; the use of needles, syringes, and other sharp objects are to be strictly limited (CBS Matrix 4.6);
- plasticware can be used in place of glassware; and
- instruments should be kept moist until decontamination.
12.14 Additional Equipment Considerations for Toxins
The following are additional equipment considerations for toxin work:
- plasticware should be used in place of glassware;
- thin-walled glassware should be avoided; and
- glass chromatography columns should be enclosed in a secondary container.
Figure 12-1: Representative Diagram of a Vacuum System Set-up for the Aspiration of Infectious Liquids
Liquid from a conical centrifuge tube is aspirated into a flask containing a disinfectant solution used for the collection and decontamination of liquid waste. This flask is connected via a hose to a second flask (also containing disinfectant) that is used to collect any overflow and trap aerosols. The vacuum source (in this illustration, a portable vacuum pump) is protected against infectious aerosols or aerosolized toxins through the use of an in-line filter (0.2 µm filter is illustrated) connected between the overflow flask and the vacuum source.
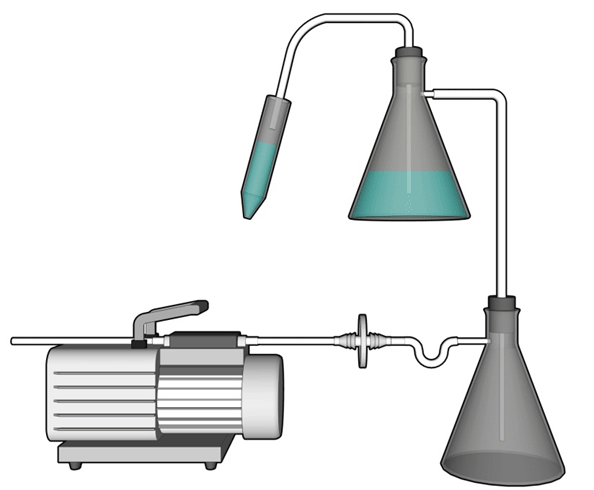
Text Equivalent - Figure 12-1
In this diagram, liquid from a conical centrifuge tube is aspirated through a tube into a conical flask containing a disinfectant solution used for the collection and decontamination of liquid waste. This flask is connected via a hose to a second flask, which also contains disinfectant, and is used to collect any overflow and to trap aerosols. The vacuum source in this illustration is a portable vacuum pump. It is protected against infectious aerosols or aerosolized toxins through the use of an in-line filter, in this case a 0.2 µm filter, connected between the overflow flask and the vacuum source.
References
- Footnote 1
- Government of Canada. (2015). Canadian Biosafety Standard (2nd ed.). Ottawa, ON, Canada: Government of Canada.
- Footnote 2
- Schmid, I., Lambert, C., Ambrozak, D., & Perfetto, S. P. (2007). Standard Safety Practices for Sorting of Unfixed Cells. Current Protocols in Cytometry. 3.6.1-3.6.20.
- Footnote 3
- Schmid, I., Roederer, M., Koup, R. A., Ambrozak, D., Perfetto, S. P., & Holmes, K. L. (2009). Biohazard Sorting. In Darzynkiewicz, Z., Robinson, P. J., & Roederer, M. (Eds.), Essential Cytometry Methods (pp. 183-204). Maryland Heights, MO, USA: Academic Press.
Chapter 13 - Animal Work Considerations
Conducting in vivo work (i.e., working with live animals) with pathogens and toxins in a containment zone increases the risk substantially compared to in vitro work. Animals can behave unpredictably, especially if they are ill. In addition, infected animals may be symptomatic or asymptomatic, or may be carriers of zoonotic pathogens also capable of causing disease in humans. Pathogens or toxins may be present in the large volumes of waste produced by animals, and can also be shed from their bodies. Exposure to pathogens that animals may harbour can occur as a result of animal bites, scratches, aerosols, or through direct contact with animal waste and bodily fluids. The risk of exposure to pathogens that animals may harbour can be reduced through an animal health surveillance program, with an emphasis on the selection of disease-free animals and the identification and treatment of diseased animals.
Additionally, some personnel may develop allergies from repeated exposure to animal fur or hair, dander, bedding, feed, and animal waste. As documented in Biological Safety Principles and Practices (2004), at least one-fifth of people who work with laboratory rodents, guinea pigs, and rabbits develop allergies. Footnote 1 An allergic reaction may manifest itself immediately or become more severe with each additional exposure. Symptoms may range from mild rashes to severe asthma. Unnecessary exposure to these allergens can be minimized through engineering controls (e.g., biological safety cabinets [BSCs], ventilated cage changing station, ventilation, use of isolators and containment caging systems), and appropriate use of respiratory protection and other personal protective equipment (PPE).
Large-sized animals also have the potential to kick, trample, or cause crushing injuries. The potential for personnel exposure to other physical hazards through equipment use and to associated noises should also be considered. The requirements for animal containment zones are specified in Chapters 3, 4, and 5 of the Canadian Biosafety Standard (CBS); animal-specific operational practice requirements are highlighted in Matrix 4.7. Footnote 2
The use of animals for experimental purposes is highly controlled and monitored. Whenever scientific research, teaching, or testing requires the use of animals, the institutional animal care committee reviews and assesses animal use protocols to ensure compliance with the Canadian Council on Animal Care (CCAC) guidelines, and, where applicable, provincial/territorial legislation specific to animals in research. The CCAC is a national peer review agency responsible for setting and maintaining standards for the ethical use and care of animals in science. The CCAC acts in the interests of Canadians to ensure that the use of animals for research, teaching, and testing employs optimal care according to acceptable scientific standards. The CCAC also promotes an increased level of knowledge, awareness, and sensitivity to relevant ethical principles. For more information on CCAC programs, please contact the CCAC or visit their website.
13.1 Animal Characteristics
Awareness and familiarity with the behavioural (i.e., instincts and mentality), psychological, and social needs of the animals by containment zone personnel, including veterinarians, scientists, and animal handlers, is fundamental in predicting how the animal(s) will act and mitigating the associated risks. For this reason, project design should include the needs of the animals with respect to their physical attributes, their susceptibility to adventitious pathogens, and the shedding and transmission of pathogens. Feeding, watering, and environmental requirements may also differ from species to species. Some animals are best housed in groups while others may require separation. Some animals will need to be observed closely to evaluate the compatibility and dynamics among the group in order to minimize fighting or injuries. In all cases, the safety of personnel is of the highest priority when evaluating animal housing options. An adaptation period for the animals (i.e., acclimatization to their new surroundings) is important to reduce initial stress and anxiety, and should, therefore, be incorporated into the experimental design. Researching the needs of the animal is essential; CCAC guidelines, literature reviews, peer-reviewed articles, and consulting with a veterinarian can provide personnel with vital information on a wide range of animal species. It is important that the animal's needs as well as the needs of the project are properly balanced in the design of the study.
The following recommendations and precautions are applicable to work with many animal species:
- Consideration should be given to the behavioural, emotional, and social needs of laboratory animals when planning their housing. For group caging, factors such as compatibility between individual animals and the population dynamics of the species should be considered in order to minimize fighting and other adverse events.
- Behavioural conditioning can be effectively used in combination with restraint procedures.
- Animal handlers should always be protected by PPE based on a local risk assessment (LRA). When appropriate, arm-length reinforced leather gloves and long-sleeved gowns or coveralls should be worn to prevent scratches.
- Protective clothing that has been in contact with animals should be decontaminated before being sent to laundry; laundering equipment located inside the containment zone is only suitable for decontamination when it has been proven to be effective for decontamination of the pathogen(s) present or suspected (i.e., validated).
- Animal handlers should immediately and thoroughly cleanse all bites, scratches, and abraded skin, and rinse all splashes that result in contact with mucous membranes. Such exposures are to be reported without delay (CBS Matrix 4.9) and post-exposure procedures implemented in accordance with the established emergency response plan (ERP) and the medical surveillance program.
- Security locks and closing devices on caging should take into consideration the persistent, creative, destructive, and intellectual capacities of the animal species (e.g., non-human primates [NHPs], raccoons), as appropriate.
- Cages should be equipped with a mechanism to facilitate examination and immobilization. Transfer boxes and other special apparatus can be used to hold animals safely while primary cages are being cleaned or to move animals from one room to another.
13.2 Animal Containment Zone Designs
An animal containment zone refers to a series of co-located animal rooms or animal cubicles, as well as associated corridors and support areas (e.g., storage rooms and preparation areas, post mortem rooms [PM rooms]) of equal containment level. The CBS specifies two types of animal containment zones: small animal containment zones (SA zones) and large animal containment zones (LA zones). It is important to note that the designation as an SA zone or LA zone is dependent on the way in which the animal is housed rather than the actual physical size of the animal. For example, if an animal containment zone houses small-sized animals, such as mice in primary containment caging, then it would be considered an SA zone. In contrast, where small-sized animals are housed in open caging only intended for the confinement of animals to an area (i.e., it does not include filtration to prevent the release of infectious materials and toxins, such as a wire cage), where aerosols generated by the animals can contaminate the room, it is then considered to be an LA zone, despite the actual size of the animal. In an LA zone, the rooms housing the animals provide primary containment (i.e., animal cubicles). Guinea pigs, rats, and mice are examples of small-sized animals that can be easily housed in filtered cages or cage rack systems in SA zones. NHPs and other large-sized animals (e.g., pigs, sheep, and raccoons) can be considered to be housed in SA zones when they are housed in primary containment caging or the caging has been completely housed inside a custom ventilated enclosure.
In addition to meeting the requirements specified in Chapters 3, 4, and 5 of the CBS, SA zones and LA zones should be designed and operated in accordance with the CCAC Guidelines on Laboratory Animal Facilities. Footnote 2Footnote 3 Institutions using animals for research, teaching, and testing should hold a CCAC Certificate of Good Animal Practice®, which is provided to facilities that have been assessed and found to have standards of experimental animal care and use that satisfy the CCAC's guidelines and policy statements.
13.2.1 Small Animal Containment Zones
Animal containment zones where animal species are housed and handled in primary containment devices (i.e., filtered containment caging and BSCs) are referred to as "small animal containment zones" (or SA zones). The room where animals are housed in primary containment caging within an SA zone is referred to as an "animal room". Figure 13-1 illustrates a basic animal room.
Many different types of primary containment caging systems are available. These can range from microisolators, to more complex models that incorporate the use of high efficiency particulate air (HEPA) filters, to completely ventilated containment caging rack systems. The type of cage selected for a project should be compatible with the animal species and the planned method of decontamination. The caging requirements and operational activities are to reflect the containment level required for the pathogen in question. Matrix 3.7 of the CBS should be consulted directly to determine the minimum requirements for primary containment caging in SA zones. Advances in caging technologies have allowed better control of microenvironmental factors such as temperature, air exchange, and humidity. Containment zone design and support systems should take into consideration the type of caging system that will be used, in order to provide appropriate backup power, humidity, and ventilation.
Figure 13-2 illustrates a ventilated caging system, commonly used in SA zones as primary containment caging. Figure 13-2(a) depicts a ventilated cage rack (containing multiple microisolator cages) that supplies a source of filtered air into the individual cages. Exhaust air is either filtered and recirculated into the room, or discharged directly into the room exhaust system. Figure 13-2(b) illustrates a microisolator cage with a filter top and connected to a filtered exhaust that provides primary containment for small-sized animals (e.g., mouse). While filters are necessary, the need for HEPA filters will depend on the pathogen (i.e., LRAs).
13.2.2 Large Animal Containment Zones
An animal containment zone where the rooms housing the animals provide the primary containment is termed a "large animal containment zone" (LA zone). The room or space inside an LA zone where animals are housed is referred to as an "animal cubicle ". Unlike a laboratory work area or SA zone, where the BSC or primary containment caging provides primary containment and the mechanical systems provide secondary containment, an animal cubicle in an LA zone provides both primary and secondary containment. Animals in an LA zone are not housed in primary containment caging (e.g., they are housed in stalls, pens, or non-filtered cages). Non-filtered cages (detailed in Figure 13-3) are a type of open caging that can be used to house small-sized animals (e.g., raccoons, NHPs).
The selection of animal housing and handling equipment should be specific to the species. For example, an LA zone can house mice, raccoons, NHPs, or dogs in non-filtered cages (i.e., caging only intended to restrict animals to an area and that does not include filtration to prevent the release of infectious material or toxins), chickens or pigs in pens, or livestock or deer housed in stalls inside a cubicle. Figure 13-4(a) illustrates an example of an animal cubicle equipped with open cages (i.e., non-filtered) suitable to house a number of animals such as dogs, cats, racoons, or NHPs; Figure 13-4(b) illustrates an animal cubicle designed with an alternative open caging system: three stalls suitable to house up to three large-sized animals such as cows, deer, horses, or sheep.
LA zones may accumulate a high concentration of pathogens in the animal cubicles, and the animals have the potential for the generation of high concentrations of infectious aerosols. Particular attention should be given to the use of PPE worn by personnel entering an animal cubicle in an LA zone, as PPE serves as the primary protection against exposure to pathogens. PM rooms are rooms inside LA zones where animal necropsies and dissections are performed, and there may be several PM rooms within an LA zone. In some cases (e.g., an LA zone where small-sized animals are housed in open caging systems), necropsies and dissections may be performed outside the LA zone in a BSC (i.e., not in a PM room). PM rooms are likely the area of greatest contamination; necropsy procedures are often associated with a high risk of generating infectious aerosols, splashes or spills of infectious material, and general gross contamination. In addition, exposures to pathogens and toxins in PM rooms may involve cutting instruments or the sharp ends of cracked bones. Similar to an animal cubicle, PPE is extremely important to protect personnel from exposure in a PM room and prevent the spread of contamination; therefore, PPE selection should take this into account. Engineering controls, such as a downdraft table, can be used to help reduce the spread of aerosols in the PM room, but will not fully contain infectious material and toxins. Entry and exit procedures in place for PM rooms include direction on sufficient time for aerosols to settle, prior to opening doors, especially where anterooms to the PM room are not provided.
Animal cubicles and PM rooms in LA zones require additional and sometimes unique physical containment and operational practices in order to contain the pathogens and toxins and protect personnel entering these spaces from exposure. Consequently, Chapters 3, 4, and 5 of the CBS specify the requirements for LA zones and distinguish the requirements for LA zones at containment level 2 (CL2) and containment level 3 (CL3) from other work areas of the same containment level by separating these requirements and labelling them as "CL2-Ag" and "CL3-Ag", respectively (i.e., "Ag" for "Agriculture").
13.2.3 Animal Containment Zone Design Considerations
Design considerations relevant for the design of any containment zone, including animal containment zones, are discussed in Chapter 22; this section discusses several key concepts relevant only to the design of animal containment zones.
13.2.3.1 Single Corridor versus Dual Corridor Designs
Where there are numerous animal rooms or cubicles within a more complex containment zone, the inclusion of separate "clean" (i.e., uncontaminated) and "dirty" (i.e., contaminated or potentially contaminated) corridors may ease personnel movement from one room or cubicle to the next. LA zones incorporating this dual corridor design (i.e., separate "clean" and "dirty" corridors) connecting the animal cubicles and PM rooms can offer advantages over LA zones designed with a single corridor connecting the animal cubicles and PM rooms ("single corridor design"). The dual corridor design facilitates traffic flow of animal handlers, staff, animals, feed, equipment, and samples/specimens. This design can also minimize the risk of cross-contamination between animal cubicles. The flow of animals and personnel in animal containment zones designed with a single corridor layout is considerably different from that in zones with a dual corridor layout. It is critical that traffic flow for animals and personnel be well defined in the standard operating procedures (SOPs) for single and dual corridor designs. Examples of single corridor and dual corridor designs in CL2 or CL3 LA zones (i.e., CL2-Ag or CL3-Ag) are provided in Figure 13-5.
A single corridor design facility may be operated in a manner that designates the corridor as a "clean" corridor (i.e., uncontaminated), in which case, anterooms for the entry to/exit from each animal cubicle and PM room are important and the strict adherence to operational procedures (especially entry/exit protocols) is critical to prevent the spread of contamination in the containment zone. Alternatively, where the single corridor is operated as the "dirty" corridor, the presence of an anteroom at each animal cubicle and operational protocols at these points is not highly emphasized since it does not separate clean and dirty areas. In this case, strict adherence to entry/exit procedures at the containment zone entry/exit is essential to prevent the release of pathogens from the zone. In the single corridor design depicted in Figure 13-5(a), the access corridor is considered "dirty" (i.e., contaminated). The containment zone is accessed by personnel through an anteroom located off the corridor (entry/exit). Each animal cubicle and PM room is accessed by personnel from the corridor via separate anterooms (entry/exit). In facilities where anterooms and showers are not available at each cubicle (e.g., CL2 LA zones), procedural means to limit contamination may be an option if supported by an LRA. Proper training of personnel in the movement between cubicles (e.g., handling uninfected animals first, using boot baths and chemical disinfection of outer PPE layers after exiting a cubicle into a "dirty" corridor) can be effective in preventing contamination.
In contrast, in the dual corridor design (Figure 13-5[b]), there are separate "clean" and "dirty" corridors to minimize the spread of contamination from infected animals to specific areas of the zone. The containment zone is accessed by personnel through an anteroom located off of the "clean" corridor (entry/exit). The "dirty" corridor allows for the movement of infected animals between cubicles and PM rooms. Animal entry to the containment zone is through the "clean" corridor. Entering more than one animal room or cubicle from the "clean" corridor, without a change of PPE, is generally not acceptable; however, in some cases, it may not be necessary to change PPE when moving from uninfected animals to infected animals. Entering more than one animal cubicle from the "dirty" corridor may be acceptable, provided that the same pathogen is handled in all rooms and cubicles and depending on the nature of the work. After personnel are finished working in animal cubicles or PM rooms, dedicated PPE is doffed in the connecting anteroom(s) before re-entering the "clean" corridor. The containment zone is exited through an anteroom located off the "clean" corridor.
13.2.3.2 Access and Anterooms
Access to animal cubicles in LA zones is provided through one or more anterooms. Depending on the design (described in Section 13.2.3.1) and containment level of the containment zone, anterooms may also be located at the entry to/exit from individual animal cubicles and PM rooms. Anterooms create an added buffer space to protect the outer environment from the infectious material and toxins handled within; they allow for the separation of personal clothing from dedicated animal cubicle clothing and PPE, and help to maintain the inward directional airflow (IDA) in animal containment zones to protect containment integrity. An LRA may be conducted to determine when a shower is needed prior to exiting from a CL2 LA zone (i.e., CL2-Ag) for example, if there is substantial contact with infected animals on a day-to-day basis, or when working with animals that harbour, as part of their normal flora, pathogens that may infect humans. Anterooms are further discussed in Chapter 3.
Restricting access to the animal containment zone increases the safety of personnel and increases the security of the animals and pathogens and toxins handled and stored inside the containment zone. In certain cases, limited access or restricted access to areas within the containment zone (e.g., individual animal rooms, animal cubicles, and PM rooms) may also be necessary and is determined by the pathogens, toxins, and activities in the zone. Controlled access systems, such as electronic access card systems, keypads, or key-locks with non-reproducible keys, restrict access to authorized personnel. Restricting access to animal rooms, animal cubicles, or PM rooms may be achieved through a controlled access system, or where determined to be acceptable, through other mechanisms such as signage (e.g., "authorized personnel only"). Observation windows on the doors accessing animal rooms and cubicles are generally recommended to allow personnel to view the interior of the animal room or cubicle prior to entry and to verify that animals are not loose.
13.2.3.3 Cold Storage
If tissues and carcasses are not disposed of immediately following euthanasia or necropsy procedures, refrigeration will be required to delay putrefaction and to minimize odours. Consideration should be given to the size of the animals in use and the quantity of carcasses that will need to be stored prior to disposal to confirm that the cold storage area or equipment needed is sufficient in size; this equipment could be an integral cold room or refrigeration equipment, such as a freezer or refrigerator, of adequate size, dependent on the size of the animals in use. Locating cold storage in or adjacent to the PM room in an LA zone will minimize the distance to move potentially heavy carcasses and limit the spread of contamination.
13.2.3.4 Unique Physical Requirements
Animals are curious by nature and have the ability to chew on or pull objects, and as such, protruding obstructions (e.g., lighting, electrical fixtures, exposed plumbing) in these spaces should be minimized and appropriately shielded. Locking mechanisms should be carefully selected to be sufficiently complex to prevent animal escape, as determined by the animal's ability to manipulate objects. In animal containment zones, floors are to be impact-resistant and able to withstand the weight of animals and associated equipment without becoming gouged or cracked (CBS Matrix 3.4). They should also be designed to withstand prolonged contact with urine. Gates, rubber mats, and cages should have sufficient strength to resist the damage and abuse caused by the animal(s). Building and surface coverings of an animal containment zone should be selected knowing that animal rooms, animal cubicles, and PM rooms are subjected to frequent cleaning, decontamination, and high pressure washing.
Floors should be textured and slip-resistant so animals and animal handlers can maintain traction, even when the surface is wet. Personnel should also wear footwear that provides traction on wet, slippery floors. Due to the large volume of water that is needed for the cleaning of these spaces, it is recommended that floors slope directly towards the floor drains to avoid pooling of contaminated water. In CL2 LA zones (i.e., CL2-Ag) where prions are handled, CL3 zones where non-indigenous animal pathogens are handled, and CL3 LA zones (i.e., CL3-Ag), floors drains are to be separated from those of lower containment areas and directly connected to an effluent decontamination system to decontaminate all liquid waste prior to release into the sanitary sewer.
13.3 Equipment
The carcasses of livestock and other large-sized animals (e.g., deer, moose) can be quite difficult to move around in the containment zone. It may be necessary to use an overhead rail and hoist system to move large carcasses to the necropsy room or disposal unit. Consideration should be given to including a rail, chain fall, motorized operation, and adequate lift clearance when planning the height of an LA zone where work with large-sized animals will be conducted. In LA zones (including PM rooms), the operation of the electrical hoist/monorail should be limited to trained personnel wearing protective headwear.
It is recommended that surgical procedures and necropsies be carried out in dedicated laboratory work areas (i.e., procedure rooms), necropsy rooms, or PM rooms located inside the animal containment zone but separate from animal rooms or cubicles, wherever possible. To preserve personnel safety and promote proper animal care, adequate preparation is crucial; all necessary tools and equipment should be available inside the containment zone. The selection of tools and equipment for use in surgical procedures and necropsies should consider the potential to cause injury to personnel and the creation of potentially infectious aerosols. For example, it may be prudent to use manual equipment (e.g., hand saw) instead of electrical equipment (e.g., Stryker saw) during these procedures so that the amount of gross contamination and aerosols is minimized. Skilful technique is required to prevent the excessive spread of contamination and the formation of aerosols originating from fluids and tissues. When performing surgical procedures and necropsies, every effort should be made to limit the spread of contamination.
13.4 Personnel Training
Personnel working with animals, facility maintenance employees, and other staff that may need to enter the facility are to have specific training in animal facility procedures (the requirements are specified in Matrix 4.3 of the CBS). Training plans are to be developed for each individual, accounting for their duties in the facility, and should include the physical and biological hazards associated with the animals themselves, restraint techniques, the characteristics of the pathogens or toxins in use, and all relevant SOPs. The relevant SOPs describe every aspect of the proposed work, including, but not limited to, entry and exit, PPE, communication between personnel, feeding, sampling, animal handling, animal escape (prevention and capture), signs of disease, daily cleaning, decontamination, surgical and necropsy procedures, and any other protocols specific to the work. The training will also include the procedures that are relevant to emergency situations, described in Chapter 17.
Development of the training program should take into consideration the applicable CCAC guidelines. Trainees may benefit from visits to other containment facilities and discussions with personnel who have extensive experience working with the animal species of interest. It is recommended that the training include mock scenarios and pre-task practice prior to the actual infection of animals. Consideration should be given to posting the contact information of experienced animal handlers throughout the animal containment zone. The training program is discussed in greater detail in Chapter 8.
13.5 Handling and Restraint
The use of proper handling and restraint techniques helps prevent injury to the handler, reducing the potential for a bite, scratch, or other exposure to infectious material, and therefore protecting against secondary transmission in the community. In addition, proper handling and restraining techniques will minimize animal stress; stressed animals are more likely to react in an unexpected manner, and from a scientific perspective, may lead to physiological changes that influence findings. Close attention should always be paid to the restraint of animals during inoculation procedures to avoid self-inoculation. Different species can have very different handling requirements. The selection of animal housing and handling equipment should be specific to the species. There are many resources available on proper handling techniques for various types of animals, including the CCAC and international resources. Footnote 4 Footnote 5 Footnote 6 Footnote 7 Footnote 8 Footnote 9 Footnote 10
When handling animals that are in caging systems, a chemical restraint or a squeeze mechanism (e.g., squeeze-back cages) may be utilized to immobilize the animals, depending on the species. Transfer boxes may be an option to move animals within the containment zone. When handling large-sized animals, special care should be taken to avoid serious injuries (e.g., crushing) that could occur. Methods such as gating systems, chutes, tunnels, squeeze mechanisms, and chemical restraint methods, can be used to restrain and move animals to other rooms or cubicles in LA zones.
Using the least amount of restraint necessary is the best way to provide a safe environment for both the animal and the handler and, when possible, it is good practice to habituate the animals to the restraint method before initiating project manipulations. For example, with positive reinforcement, pigs will habituate to a sling, and horses and cattle can be trained to accept a halter and lead rope.
13.6 Decontamination and Waste Management
The safe and effective decontamination of all waste, including animal waste, is critical to containment. In high containment LA zones (i.e., CL3-Ag and containment level 4 [CL4]), as well as in CL2 LA zones (i.e., CL2-Ag) where prions are handled, floor drains are connected to an effluent decontamination system. In contrast, in CL3 SA zones where only human or indigenous animal pathogens are handled, contaminated liquid effluent (e.g., from cage washing) is prevented from entering the floor drains procedurally. It is recommended that floor drains only be installed when necessary to minimize the potential for contaminated liquids to be released into the sanitary sewers. Where existing floor drains are not to be used, they should be sealed or capped. In all containment zones, contaminated liquids are to be decontaminated prior to release to sanitary sewers; therefore, procedures that reduce the amount of liquid waste produced (e.g., use of footbaths rather than rinsing boots, capturing liquids in bedding) are encouraged.
In animal cubicles connected to the effluent decontamination system, the drains can potentially become clogged by bedding or litter. Therefore, effluent decontamination systems are to include a mechanism to prevent blockage. This mechanism may be physical or operational, such as regularly removing bedding or litter from the drains and having them autoclaved or incinerated, provided that validation and efficacy testing demonstrate the approach to be effective. Decontamination of animal carcasses and anatomical waste is further described in Chapter 15.
A variety of equipment and processes can be used for cage cleaning. In SA zones, cage manipulations and bedding disposal are performed in a BSC or ventilated cage changing station designed to contain aerosols and provide user and environmental protection. Non-ventilated animal transfer stations should not be used when handling infected animals as they offer no environmental or user protection. The cages should be closed and surface decontaminated before removal from the BSC, and then sent for autoclaving before final cleaning. Various types of caging and bedding disposal systems exist and thorough research is essential to select an appropriate system.
Cage washers can only be used as the primary decontamination technology if the method has been validated to be effective against the pathogens or toxins in use. Often, cages and bedding are decontaminated inside the containment zone, before they are sent for cage washing. In high containment zones, cage washing areas can be located outside of the containment zone only if cages are decontaminated prior to removal from the containment zone. The use of disposable caging is an alternative to having cage washing areas.
The use of pressure washers to clean animal cubicles should be minimized and used only when necessary in order to prevent the generation of aerosols. Cubicles can first be cleaned with low pressure hoses and then sprayed with a pressure washer. Cleaning cubicles on a daily basis is recommended in order to reduce the accumulation of contaminants, with complete decontamination of the cubicle being carried out at the end of the experiment.
13.7 Confinement
Confinement is the term used when only certain containment components are required. During specific periods of time subsequent to inoculation with certain pathogens, natural excretions and casual contact with infected animals would not pose a risk for pathogen transmission (e.g., during the incubation period for bovine spongiform encephalopathy [BSE] in cattle). Thus, while the infected animals should always remain adequately confined, they do not have to be housed and maintained inside a containment zone as specified in the CBS. Confinement components will vary depending on the pathogens used and the study design. Many factors need to be taken into consideration, including disease transmission, potential for shedding, and endemicity in Canada. Due to recent evidence that scrapie can be detected in the feces and oral secretions of pre-clinical sheep through sensitive techniques, it is not acceptable to house scrapie-infected sheep in confinement at any time post-infection or exposure; they must be housed in an appropriate containment zone. Footnote 11Footnote 12Footnote 13
Approval by the Public Health Agency of Canada (PHAC) and/or the Canadian Food Inspection Agency (CFIA) is required before experimentally infected animals are permitted to be housed in confinement conditions. The basic and minimal confinement components needed are based on the results of an LRA. Some additional considerations when working in confinement are as follows:
- Observation and counting of animals in confinement should be performed and recorded daily. Single-point access control is recommended in order to prohibit unauthorized movement of personnel or animals into or out of the confinement area. Access control should be verified to make sure it functions as intended.
- A method to limit the access of wildlife/scavengers to the confinement area should be put in place.
- A double identification (ID) system for animals should be in place and verification of ID should be conducted daily. If an ID tag or other device is missing it should be replaced immediately.
- Materials (e.g., manure and bedding) from pens may be treated by normal composting and disposal. Composting parameters require validation testing to demonstrate efficacy against the infectious material in use.
- Animals that have been inoculated with human or animal pathogens, or other experimental biological material, are not eligible for use in the human food or animal feed chain.
13.8 Special Considerations for Work with Prion-Infected Animals
Prions associated with transmissible spongiform encephalopathies (TSEs) can infect a wide range of animal species, including cattle, sheep, deer, and mink, as well as humans. While there do not appear to be any confirmed cases of laboratory acquired infections (LAIs) of TSEs in humans, precautions are required when working with prions and animals infected with prions to avoid worker exposure to contaminated materials and release of infectious prions into the environment. The most likely routes of prion transmission to humans are through accidental inoculation with contaminated instruments, bites or scratches from infected animals, or ingestion of material containing prions. There is little evidence to suggest that prion diseases are transmissible by inhalation; however, transmission through exposure to aerosols or splashes may be possible, especially if there is contact with the mucous membranes. Footnote 14 Footnote 15 Footnote 16 Footnote 17
To date, little evidence has been found to suggest that either animal to animal or maternal transmission of BSE occurs amongst cattle in a herd; rather, transmission appears to occur through the ingestion of contaminated feed (meat and bone meal). Footnote 18Footnote 19Footnote 20Footnote 21Footnote 22 In contrast, maternal transmission as well as animal to animal transmission of scrapie have been documented amongst sheep of the same herd. Footnote 14 Footnote 23 Footnote 24 Transmission of chronic wasting disease (CWD) in deer and elk has been documented to occur through both animal to animal contact as well as by contact with a heavily contaminated environment. Prions have been detected in many types of cervid tissues (e.g., central nervous system tissue, blood, cardiac and skeletal muscles, pancreas) as well as in bodily secretions and excretions (e.g., feces, urine, saliva) of CWD-infected cervids. Footnote 25Footnote 26
Given the knowledge of prion transmission and the lengthy incubation period of TSEs in hosts, some animals inoculated with prions only present a significant risk for pathogen transmission during certain periods (i.e., immediately after inoculation, parturition) when prions may be shed in bodily secretions and excretions. The following operational practices are considerations when working with animals infected with prions and disposing of wastes:
- For BSE in cattle: Waste and bedding should be collected and treated accordingly for potential prion contamination for at least 4 weeks post-inoculation and again once clinical signs are observed. During the periods of incubation (i.e., more than 4 weeks post-inoculation and prior to the onset of clinical signs), natural excretions and casual contact with infected animals is not considered to pose a risk for prion transmission. Consequently, during these non-shedding periods, it may be acceptable (i.e., with prior approval from the PHAC or the CFIA) to house the animals outside of the CL2 LA zone (i.e., CL2-Ag), provided that they are adequately confined, based on an LRA of specific experiments. During confinement, bedding and waste may be dealt with by normal composting and disposal.
- For scrapie in sheep: Bedding, placental fluids, and any other waste should be collected and decontaminated appropriately to inactivate prions for at least 4 weeks post-inoculation and again once clinical signs are observed. Experiments should be conducted in a CL2 LA zone (i.e., CL2-Ag) suitable for work with prions while lambing. Pregnant ewes should be contained so that placental materials may be collected and incinerated and birthing fluids appropriately decontaminated. Lambing areas should be decontaminated upon removal of the ewe and lamb.
- For CWD in cervids (e.g., deer, elk, and moose): CWD-infected animals must remain in an appropriate containment zone at all times post-inoculation. All waste and bedding must be collected and decontaminated throughout the entire study period.
- For uncharacterized TSEs (e.g., Feline Spongiform Encephalopathy, Exotic Ungulate Spongiform Encephalopathy) and non-host species inoculated with a prion disease agent (e.g., CWD in cattle or BSE in deer): In cases where it may be unclear whether or not prions are shed in animal waste, all animal waste and bedding (at all times post-inoculation) is to be collected and decontaminated throughout the entire study period. Incineration at 850°C is recommended for decontamination of solid animal waste and bedding that contains prions. An acceptable method to decontaminate liquid wastes that contain prions is heat treatment at 134 °C for 1 hour.
Additional details on the recommended methods of decontamination of wastes and other prion-contaminated materials are discussed in Chapter 15.
13.9 Working with Non-Human Primates
Work with NHPs presents unique hazards to animal handlers and containment zone personnel. Not only may NHPs harbour pathogens (i.e., normal flora) that can affect the health and safety of personnel, but the animals themselves also pose a risk. For example, the physical characteristics of NHPs (e.g., canine teeth, powerful jaws, sharp fingernails and toenails), make them capable of causing serious injury to animal handlers that may also result in an exposure to pathogens. These characteristics should be considered when designing animal rooms or cubicles to house them. The CCAC Guidelines provide information on housing and handling requirements specific to NHPs. Footnote 6
Pathogens that may be naturally carried by NHPs and pose a hazard to personnel handling them include, but are not limited to, bacteria (e.g., Salmonella, Shigella, Campylobacter, Mycobacterium tuberculosis), viruses (e.g., hepatitis A virus, simian immunodeficiency virus, Macacine herpesvirus 1 [formerly known as herpes B virus or cercopithecine herpes virus 1]), and protozoan and metazoan parasites (e.g., Entamoeba, Blastocystis, Trichomonas, Balantidium). Macacine herpesvirus 1 is an enzootic virus present in up to 70% of captive macaques, including rhesus and cynomolgus macaques. Footnote 27 Although the virus causes oral lesions in its natural simian host, asymptomatic shedding from the buccal mucosa and urogenital tract, although rare, or the presence of the virus in conjunctival fluid, can occur without clinical signs of the disease. Human infection has been documented in at least 50 instances, resulting in either severe disease or death. Footnote 28 Except for one case of person-to-person transmission, all have occurred in people exposed to NHPs or NHP tissues. Transmission to humans is believed to occur primarily by exposure to contaminated NHP saliva through bites and scratches, although one fatal case following mucocutaneous exposure without injury has been reported. Footnote 27 Guidelines are available for working safely with macaques, for the prevention of Macacine herpesvirus 1 infection, and for the treatment of such infections in exposed people. Footnote 5 Consequently, it is strongly encouraged that all macaque colonies are handled with caution and as potential carriers of Macacine herpesvirus 1, even if they have been tested seronegative for the antibody.
Except when an NHP has been experimentally infected with or known to be naturally harbouring an infectious organism requiring high containment (i.e., CL3 or higher), it is acceptable and considered safe to handle NHPs in a CL2 animal containment zone, provided that the additional precautions outlined in Section 13.1 and the following additional practices and precautions outlined below are also implemented:
- any NHP that has caused a bite or scratch wound should be immediately immobilized and have its oral cavity examined for lesions characteristic of Macacine herpesvirus 1;
- protection against aerosol exposure and splashes onto mucous membranes (e.g., with surgical mask, face shield, eye goggles) should be provided for handlers and anyone entering animal cubicles where NHPs are housed; and
- an emergency medical contact card must be issued to containment zone personnel handling NHPs; details are provided in Chapter 7.
Figure 13-1: Representative Diagram of a Basic Animal Room
Ventilated cage racks and primary containment cages are shown in the inset.
Text Equivalent - Figure 13-1
This figure depicts a 3D rendering of an animal room in which two wheeled ventilated cage racks can be seen. The inset shows a close-up view of a ventilated cage rack and a primary containment cage with a filter top.
Figure 13-2: Representative Diagrams of Primary Containment Caging
(a) A ventilated cage rack (containing multiple microisolators). Exhaust air is either filtered and recirculated into the room or discharged directly into the room exhaust system; (b) A single ventilated microisolator cage with a filter top. When connected to a ventilated cage rack system to exhaust and filter the air from the cage, this type of cage provides primary containment for small-sized animals.
(a) Rack containing multiple primary containment cages

Text Equivalent - Figure 13-2a
Figure 13-2(A) depicts a more detailed view of an example of a ventilated caging system. The system consists of a ventilated cage rack that supplies a source of filtered air into the individual cages, or microisolators. The cage rack has a ventilation system and a grid-like frame into which the individual cages are inserted.
(b) A single primary containment cage

Text Equivalent - Figure 13-2b
Figure 13-2(B) depicts a mouse in a clear, plastic, ventilated microisolator cage with HEPA-filtered exhaust. This apparatus provides primary containment for small-sized animals such as mice.
Figure 13-3: Representative Diagram of an Open Caging System
Detailed illustration of a typical wire cage (non-filtered) used to house small-sized animals (e.g., NHPs or raccoons) in an animal cubicle. This type of cage confines the animal to a small space within the cubicle.

Text Equivalent - Figure 13-3
This figure provides a detailed illustration of a typical wire cage (non-filtered) used to house small-sized animals, such as nonhuman primates or raccoons, in an animal cubicle. This type of cage confines the animal to a small space within the cubicle but provides no containment of pathogens.
13-4: Representative Diagrams of an Animal Cubicle
(a) Animal cubicle containing multiple open cages (non-filtered). This configuration is suitable to house animals such as dogs, cats, racoons, or NHPs. (b) Animal cubicle equipped with stalls and gating systems. This configuration is suitable to house up to three large-sized animals, such as cows, deer, horses, or sheep.
(a) Animal cubicle containing multiple open cages (non-filtered)
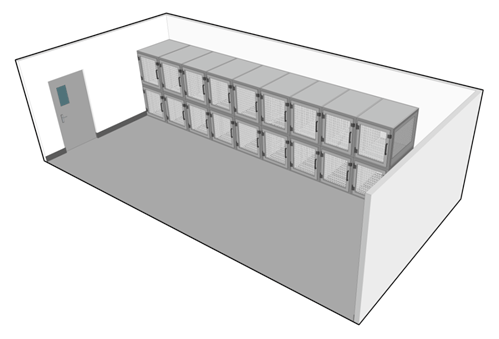
Text Equivalent - Figure 13-4a
Figure 13-4(a) depicts a 3D rendering of an animal cubicle containing multiple open cages (non-filtered wire cages) along one wall of the cubicle. This configuration is suitable to house animals such as dogs, cats, racoons, or nonhuman primates.
(b) Animal cubicle equipped with stalls and gating systems
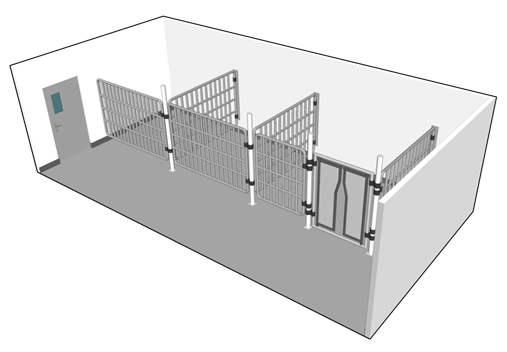
Text Equivalent - Figure 13-4b
Figure 13-4(a) depicts a 3D rendering of an animal cubicle containing multiple open cages (non-filtered wire cages) along one wall of the cubicle. This configuration is suitable to house animals such as dogs, cats, racoons, or nonhuman primates.
Figure 13-5: Representative Diagram of Single Corridor and Dual Corridor Designs for Animal Containment Zones
(a) Single corridor design for a CL2 or CL3 LA zone, (i.e., CL2-Ag or CL3-Ag); (b) Dual corridor design for a CL2 or CL3 LA zone, (i.e., CL2-Ag or CL3-Ag). Note that for CL2 LA zones, an anteroom is only required at the entry/exit to the containment zone or into each animal cubicle or PM room. For more detail, refer to Section 13.2.3.
(a) Single corridor design, CL2 and CL3 LA zones
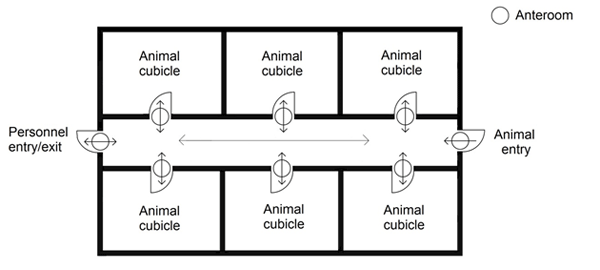
Text Equivalent - Figure 13-5a
Figure 13-5(a) features a single corridor design of an animal containment zone appropriate for CL2-Ag or CL3-Ag. The single corridor across the middle is considered "dirty". The containment zone is accessed by personnel through an anteroom located off the corridor (entry/exit). The entry of infected animals into the containment zone is through a separate anteroom located off the corridor. Each animal cubicle and PM room within the containment zone is accessed by personnel from the corridor via separate anterooms (entry/exit).
(b) Dual corridor design, CL2 and CL3 LA zones
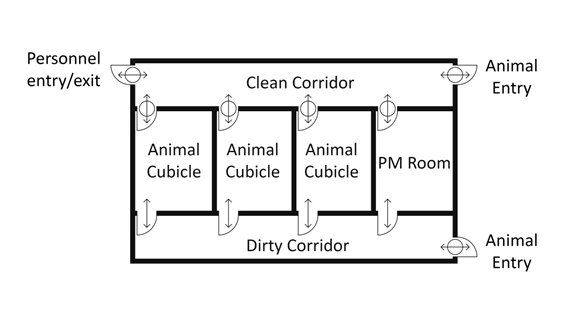
Text Equivalent - Figure 13-5b
Figure 13-5(b) depicts a dual corridor design for a CL2-Ag or CL3-Ag area where there are separate "clean" and "dirty" corridors. In this layout, animal cubicles and post-mortem rooms are located between a clean and dirty corridor. The entry and exit of personnel and uninfected animals into the containment zone occurs through anterooms located off of the "clean" corridor. Infected animals may only enter the containment zone from an anteroom located off of the "dirty" corridor. Each animal cubicle and PM room within the containment zone is accessed through an anteroom off of the "clean" corridor. There are also entry/exit points from the "dirty" corridor to each animal cubicle and PM room.
Note that for CL2-Ag, an anteroom is only required at one of the following; either at the entry/exit to the containment zone, or into each animal cubicle or PM room. For more detail, refer to Section 3.7.
References
- Footnote 1
- Phipatanakul, W., & Wood, R. A. (2004). Allergens of Animal and Biological Systems. In Fleming, D. O., & Hunt, D. L. (Eds.). Biological safety: Principles and practices (4th ed., pp. 241-251). Washington, DC, USA: ASM Press.
- Footnote 2
- Government of Canada. (2015). Canadian Biosafety Standard (2nd ed.). Ottawa, ON, Canada: Government of Canada.
- Footnote 3
- Canadian Council on Animal Care. (2003). CCAC Guidelines on: Laboratory Animal Facilities - Characteristics, Design and Development. Ottawa, ON, Canada: Canadian Council on Animal Care.
- Footnote 4
- Canadian Council on Animal Care. (1984). Guide to the Care and Use of Experimental Animals (volume 2). Ottawa, ON, Canada: Canadian Council on Animal Care.
- Footnote 5
- Olfert, E. D., Cross, B. M., & McWilliam, A. A. (Eds.). (1993). Guide to the Care and Use of Experimental Animals (2nd ed., volume 1). Ottawa, ON, Canada: Canadian Council on Animal Care.
- Footnote 6
- Canadian Council on Animal Care. (2014). Three Rs Microsite: Care and Techniques. Retrieved 03/11, 2015 from http://3rs.ccac.ca/en/care-and-techniques/ct-procedures/
- Footnote 7
- Canadian Council on Animal Care. (2009). CCAC Guidelines on: the Care and Use of Farm Animals in Research, Teaching, and Testing. Ottawa, ON, Canada: Canadian Council on Animal Care.
- Footnote 8
- National Research Council of the National Academies. (2011). Guide for the Care and Use of Laboratory Animals (8th ed.). Washington, DC, USA: The National Academy Press.
- Footnote 9
- Federation of Animal Science Societies. (2010) Guide for the Care and Use of Agricultural Animals in Research and Training (3rd ed.). Retrieved 11/03, 2015 from http://www.fass.org/docs/agguide3rd/Ag_Guide_3rd_ed.pdf
- Footnote 10
- Hubrecht, R. C., & Kirkwood, J. (Eds.). (2010). The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals (8th Ed.). Chichester, UK: Wiley-.Blackwell.
- Footnote 11
- Maddison, B. C., Rees, H. C., Baker, C. A., Taema, M., Bellworthy, S. J., Thorne, L., Terry, L. A., & Gough, K. C. (2010). Prions are secreted into the oral cavity in sheep with preclinical scrapie. Journal of Infectious Diseases. 201:1672-1676.
- Footnote 12
- Terry, L. A., Howells, L., Bishop, K., Baker, C. A., Everest, S., Thorne, L, Maddison, B., & Gough, K. C. (2011). Detection of prions in the faeces of sheep naturally infected with classical scrapie. Veterinary Research. 42:65-71.
- Footnote 13
- Gough, K. C., Baker, C. A., Rees, H. C., Terry, L. A., Spiropoulos, J., Thorne, L., & Maddison, B. C. (2012). The oral secretions of infectious scrapie prions occurs in preclinical sheep with a range of PRNP genotypes. Journal of Virology. 86:566-571.
- Footnote 14
- Denkers, N. D., Seelig, D. M., Telling, G. C., & Hoover, E. A. (2010). Aerosol and nasal transmission of chronic wasting disease in cervidized mice. Journal of General Virology. 91:1651-1659
- Footnote 15
- Prusiner, S. B. (2004). Prion Biology and Diseases (2nd ed.). Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press.
- Footnote 16
- Ryder, S., Dexter, G., Bellworthy, S., & Tongue, S. (2004). Demonstration of lateral transmission of scrapie between sheep kept under natural conditions using lymphoid tissue biopsy. Research in Veterinary Science. 76(2204):211-217.
- Footnote 17
- Sigurdson, C. J., & Miller, M. W. (2003). Other animal prion diseases. British Medical Bulletin. 66:199-212.
- Footnote 18
- Smith, P. G., & Bradley, R. (2003). Bovine spongiform encephalopathy (BSE) and its epidemiology. British Medical Bulletin. 66:185-198.
- Footnote 19
- Castilla, J., Brun, A., Díaz-San Segundo, F., Salguero, F. J., Gutiérrez-Adán, A., Pintado, B., Ramírez, M. A., del Riego, L., & Torres, J. M. (2005). Vertical Transmission of Bovine Spongiform Encephalopathy Prions Evaluated in a Transgenic Mouse Model. Journal of Virology. 79:8665-8668.
- Footnote 20
- European Commission. (1999). The Possible Vertical Transmission of Bovine Spongiform Encephalopathy (BSE): Report of the Working Group. Retrieved 11/03, 2015 from http://ec.europa.eu/food/fs/sc/ssc/out44_en.pdf
- Footnote 21
- Wilesmith, J. W., & Ryan, J. B. (1997). Absence of BSE in the offspring of pedigree suckler cows affected by BSE in Great Britain. Veterinary Record, 141:250-251.
- Footnote 22
- Wrathall, A. E., Brown, K. F., Sayers, A. R., Wells, G. A., Simmons, M. M., Farrelly, S. S., Bellerby, P., et al. (2002). Studies of embryo transfer from cattle clinically affected by bovine spongiform encephalopathy (BSE). Veterinary Record, 150:365-378.
- Footnote 23
- United States Department of Agriculture Animal and Plant Health Inspection Service. (2004). APHIS Factsheet: Scrapie. Retrieved 11/03, 2015 from http://www.aphis.usda.gov/animal_health/animal_diseases/scrapie/downloads/fs_ahscrapie.pdf
- Footnote 24
- Garza, M. C., Fernandez-Borges, N., Bolea, R., Badiola, J. J., Castilla, J., & Monleon, E. (2011). Detection of PrPres in Genetically Susceptible Fetuses from Sheep with Natural Scrapie. PLoS One. 6(12):e27525
- Footnote 25
- Sigurdson, C. J. (2008). A prion disease of cervids: Chronic wasting disease. Veterinary Research. 39:41.
- Footnote 26
- John, T. R., Schätzl, H. M., & Gilch, S. (2013). Early detection of chronic wasting disease prions in urine of pre-symptomatic deer by real-time quaking-induced conversion assay. Prion. 7(3):253-258.
- Footnote 27
- United States Centers for Disease Control and Prevention. (1998). Fatal Cercopithecine Herpesvirus 1 (B Virus) Infection Following a Mucocutaneous Exposure and Interim Recommendations for Worker Protection. MMWR. Morbidity and Mortality Weekly Report. 47(49):1073-1076, 1083.
- Footnote 28
- Cohen, J., Davenport, D. S., Stewart, J. A., Deitchman, S., Hilliard, J. K., Chapman, L. E., & B Virus Working Group. (2002). Recommendations for Prevention of and Therapy for Exposure to B Virus (Cercopithecine Herpesvirus 1). Clinical Infectious Diseases. 35:1191-1203.
Chapter 14 - Large Scale Work
Large scale production facilities such as industrial fermentation and vaccine production plants pose an increased risk to personnel and the environment due to the large quantities of infectious material or toxins being handled. As such, there are sometimes unique or more stringent requirements and additional considerations when compared to laboratory work areas at the same containment level. This chapter provides specific guidance to assist large scale production areas in developing a comprehensive biosafety program.
14.1 Scope
There is currently no universally accepted definition of "large scale". The United States National Institutes of Health (NIH) Guidelines for Research Involving Recombinant DNA Molecules considers anything greater than 10 litres as large scale. Footnote 1 The United States Centers for Disease Control and Prevention (CDC)/NIH Biosafety in Microbiology and Biomedical Laboratories, 4th Edition, 1999 defines "production quantities" as a volume or concentration of infectious organisms considerably in excess of those used for identification and typing. Footnote 2 In contrast, the United Kingdom Advisory Committee on Dangerous Pathogens states that it is not the volume but the intent of the work that determines the scale. Footnote 3
The Public Health Agency of Canada (PHAC) and the Canadian Food Inspection Agency (CFIA) generally consider activities involving volumes of toxins or the in vitro culture of infectious material on a scale of 10 litres or greater to be large scale. This could be a single vessel with a volume of 10 litres or greater, or in some cases, multiple vessels with a total volume of 10 litres or greater. Determination of cut-off values for laboratory and large scale volumes can be made in consultation with the PHAC or the CFIA.
14.2 Considerations for Large Scale Work
When working in a large scale environment, a local risk assessment (LRA) is conducted to identify and examine the hazards associated with the infectious material or toxins, processes, and equipment in use. This analysis is used to develop safe work practices. Once an LRA has been completed, exemptions from certain large scale requirements may be determined in consultation with the PHAC or the CFIA. In addition, elements specific to large scale activities may apply to licences and animal pathogen import permits, for certain containment level 2 (CL2) large scale production areas; this is determined based on the processes and type of pathogens used, and in consultation with the PHAC or the CFIA.
Some factors that should be considered when conducting an LRA are as follows:
- the infectious material or toxins handled (e.g., properties, risk group, containment level);
- the nature of the final biological product (e.g., live versus, attenuated virus, or inactivated pathogen component);
- the volume (i.e., total volume; single vessel versus multiple vessels);
- the concentration;
- the manipulations to be performed (e.g., in-process sampling, harvesting of cultures, concentrating, blending, interventions prior to inactivation);
- the type of process used (i.e., batch versus continuous);
- equipment characteristics (e.g., type, open or closed system for production and processing, stationary versus movable, aerosol generating); and
- facility features (e.g., climatic conditions, air supply intake and exhaust, maintenance of differential air pressures, physical security).
14.3 Fermenters
Fermenters can vary significantly in size, design, instrumentation and features, such as automation and capacity for in situ cleaning and decontamination. Infectious material or toxins could potentially be released from many areas in large scale fermentation equipment (e.g., motor shaft, exhaust gas vents, sampling ports). Fermentation processes also have the potential to generate aerosols, thereby increasing the risk associated with exposure to aerosols from infectious material or toxins. To minimize the probability of leaks and the release of aerosols when using large scale fermentation equipment, the following should be considered:
- double mechanical seals on the motor shaft should be used, or alternatively, a top-mounted agitator;
- exhaust vents should be equipped with a high efficiency particulate air (HEPA) filter, incinerator, or equivalent method of preventing pathogen release;
- sampling ports should be fitted to a sterilizable closed sampling system;
- validation of the relief system should be conducted and consideration should be made to the consequences of discharge; and
- anti-foam product is recommended to prevent blockage of the exhaust air vent.
14.4 Regulatory Considerations
The production of regulated biological products, such as vaccines and biopharmaceuticals, for human and veterinary use may require higher standards than those specified in the Canadian Biosafety Standard (CBS), 2nd Edition, 2015 to achieve the necessary product quality. Footnote 4Footnote 5 For example, additional requirements may apply to work involving veterinary biologics, including vaccines and in vitro diagnostic test kits for the detection of animal pathogens. The CFIA's Canadian Centre for Veterinary Biologics (CFIA-CCVB) is the national authority responsible for regulating veterinary biologics in Canada. In addition, Health Canada's Biologics and Genetic Therapies Directorate (BGTD) is the Canadian federal authority that regulates biological drugs and radiopharmaceuticals intended for human use. The CFIA-CCVB and Health Canada's BGTD should be consulted for any large scale exemptions for veterinary biologics intended for animal use and biological drugs/radiopharmaceuticals intended for human use, respectively. For more information regarding the regulatory requirements for veterinary biologics intended for animal use and biological drugs and radiopharmaceuticals intended for human use, please contact the CFIA- CCVB or Health Canada's BGTD directly, or visit their website.
References
- Footnote 1
- United States Department of Health and Human Services, United States National Institutes of Health. (2013). NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines). Bethesda, MS, USA: United States National Institutes of Health.
- Footnote 2
- United States Department of Health and Human Services, United States Centers for Disease Control and Prevention, & United States National Institutes of Health. (1999). Biosafety in Microbiological and Biomedical Laboratories (4th ed.). Washington, DC, USA: United States Government Printing Office.
- Footnote 3
- Advisory Committee on Dangerous Pathogens. (1998). The Large-Scale Contained Use of Biological Agents. Suffolk, UK: Health and Safety Executive / HSE Books.
- Footnote 4
- Health Canada. (2002). Guidance for Industry: Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients. ICH Topic Q7A. Ottawa, ON, Canada: Published by authority of the Minister of Health. Retrieved 11/03, 2015 from http://www.hc-sc.gc.ca/dhp-mps/compli-conform/legislation/gazette1-q7a-eng.php
- Footnote 5
- Government of Canada. (2015). Canadian Biosafety Standard (2nd ed.). Ottawa, ON, Canada: Government of Canada.
Chapter 15 - Decontamination
It is a basic biosafety principle and a critical component of containment that all contaminated material is decontaminated prior to disposal. The principles of sterilization, disinfection, and decontamination are critical for reducing the risk of pathogen release within containment zones, to the environment, and within the community. Examples of decontamination systems that may be used to decontaminate materials leaving the containment zone include, but are not limited to, autoclaves, effluent decontamination systems, incinerators, irradiators, dunk tanks, tissue digesters, and chemical showers. The requirements for decontamination procedures are described in Matrix 4.8 of the Canadian Biosafety Standard (CBS), 2nd Edition. Footnote 1
Regulated parties that properly package and label biohazardous waste for off-site decontamination by a third party biohazardous waste disposal facility remain accountable for the waste until its decontamination, including validation and verification of the decontamination process.
15.1 Principles of Sterilization, Disinfection, and Decontamination
Sterilization is a process that completely eliminates all living microorganisms, including bacterial spores. The probability of a microorganism surviving a sterilization process is considered to be less than one in one million (i.e., 1:106), and is referred to as "sterility assurance". Footnote 2 Sterilization is considered to be absolute (i.e., there is no middle range of sterility). Given that toxins and prions are not living microorganisms, the concept of sterilization does not apply. Decontamination of toxins and prions is discussed in Sections 15.11 and 15.12 of this chapter, respectively.
Disinfection is a process that eliminates most forms of living microorganisms but is less lethal than sterilization. The effectiveness of the disinfection process is affected by a number of factors, including the nature and quantity of microorganisms, the amount of organic matter present, the type and state of items being disinfected, and the temperature.
Decontamination is the process by which materials and surfaces are rendered safe to handle and reasonably free of microorganisms, toxins, or prions. The primary objective of decontamination is to protect containment zone personnel and the community from exposure to viable pathogens and toxins that may cause disease. Depending on the situation (i.e., pathogen or toxin in use), effective decontamination may require disinfection, inactivation, or sterilization to be considered safe and reasonably free of microorganisms, toxins, or prions. Decontamination procedures represent a critical element of containment; failure in the procedures can result in occupational exposure to, or the unintentional release of, infectious material or toxins. Footnote 3 Footnote 4 Footnote 5 The following are considerations for containment zone personnel responsible for developing decontamination processes and methods:
- Disinfectants effective against the infectious material in use, and neutralizing chemicals effective against the toxins and prions in use, are to be available in the containment zone and used for contaminated or potentially contaminated material, including equipment, specimen and sample containers, surfaces, rooms, and spills.
- Decontamination parameters (e.g., time, temperature, chemical concentration, humidity) consistent with the technology or method used are to be validated to demonstrate they are effective against the infectious material and toxins of concern under the conditions present.
- Prions and toxins can be resistant to the chemical disinfectants commonly used to effectively decontaminate microorganisms due to their proteinaceous nature. When working with prions and toxins, a neutralizing chemical capable of denaturing and inactivating the toxins or prions is needed for effective decontamination in the containment zone.
- Clear and strict procedures are to be in place to support routine decontamination and routine verification of the decontamination process.
- Decontamination processes and methods are to be conducted in accordance with applicable federal, provincial or territorial, and municipal regulations.
- Decontamination procedures are to be included in personnel training on the hazards and exposure/release mitigation strategies associated with the work being done. This includes information on the products used, and the factors influencing their effectiveness.
15.2 Validation and Verification of Decontamination Technologies and Processes
15.2.1.1 Validation
Autoclaves, effluent decontamination systems, and other decontamination technologies and processes are validated prior to implementation of the procedure. Validation demonstrates that the equipment and method are effective at decontaminating, inactivating, or removing the specific pathogen(s) or toxin(s) to be handled and stored. It is inferred that a validated method is suitable for its intended purpose.
Biological indicators or parametric monitoring devices (e.g., thermocouples, for heat-based technologies and processes only) can be used to confirm that treatment parameters have been achieved throughout a representative load. Placing thermocouples or indicators at various locations throughout the representative load or decontamination vessel will enable conditions in different parts of the load to be monitored. In autoclaves, they can be used to confirm that the centre of the load has achieved the temperature and time parameters necessary for successful decontamination.
The selection of an appropriate biological indicator is critical so that the resistance of the test organism adequately represents the resistance of the pathogens handled in the containment zone. In general, Geobacillus stearothermophilus spores are adequate for heat-based technologies and processes, whereas Bacillus subtilis spores can be used to validate chemical-based technologies and processes. In cases where biological or chemical indicators are not appropriate (e.g., prions), parametric monitoring devices, such as thermocouples or gauges that capture cycle time, temperature, and pressure, can be used to accurately monitor the performance of the decontamination equipment.
Validation of all decontamination technologies and processes is required prior to initial use and whenever significant changes are implemented or new pathogens are introduced so that decontamination procedures and standard operating procedures (SOPs) can be established, amended, or updated as necessary (CBS Matrix 4.8). Validation through the use of representative loads is required annually (CBS Matrix 5.1). Performing validation tests on non-contaminated representative loads that simulate a batch of materials of similar type (e.g., gloves, plastics, liquids, reusable personal protective equipment [PPE]) and quantity (i.e., number of items or size) that will be regularly processed allows an operator to place indicators safely to demonstrate that appropriate decontamination parameters are achieved throughout the load (e.g., in the bottom, middle, and top of the batch of materials). By demonstrating this with a representative load, it can be extrapolated that similar conditions are achieved in a routine load (i.e., contaminated waste) of similar type and quantity.
15.2.1.2 Verification
Once effective decontamination parameters have been established through validation, it is important that decontamination processes and procedures be monitored (verified) on a regular basis to confirm that established parameters have been met.
Verification is the routine monitoring of equipment and processes to ensure they are functioning properly and continue to meet the parameters established during validation. This can be accomplished using parametric monitoring devices, biological indicators, chemical indicators, or chemical integrators. The information captured during verification should include the cycle parameters (e.g., temperature, time, and chemical concentration), a description of the size and type of load (e.g., reusable PPE, solid waste, and liquid waste), and a short description of the procedure. For each run performed, the parameters captured may include time and temperature charts and biological indicator results. If a biological indicator is used, results of a positive control from the same lot should also be captured. A local risk assessment (LRA) will help determine the procedures for routine monitoring (e.g., daily, weekly, monthly), taking into consideration the frequency of use.
15.2.1.3 Indicators, Integrators and Parametric Monitoring Devices
A biological indicator is a standardized population of bacterial spores used to demonstrate effective sterilization conditions in a waste load. Achieving the target level of reduction in viable spores indicates that the decontamination process was effective. Attention must be paid to appropriate selection of indicators, as their design and construction vary depending on the intended use (e.g., liquid versus dry load, self-contained system, enzyme-based rapid method); the indicator should be representative of the pathogen or toxin being decontaminated.
Chemical indicators are meant to be used in conjunction with biological indicators and physical monitors (i.e., pressure and temperature gauge readings). They are used to monitor one or more parameters, but not all parameters needed for effective decontamination. Chemical indicators include autoclave tape, labels, and pouches embedded with a thermochromic ink (e.g., Bowie-Dick test packs). They provide instant results for day-to-day monitoring indicating that a certain parameter (e.g., temperature, steam, gas exposure) has been reached, but they are not an indicator of decontamination efficacy.
Chemical integrators are a form of chemical indicator used to confirm that all decontamination parameters have been met (e.g., temperature, pressure, and time for an autoclave cycle).
A comprehensive overview of biological and chemical/physical indicators and their recommended use can be found in Developing Indicators for Monitoring Sterilization in W.A. Rutala's Disinfection, Sterilization and Antisepsis in Health Care. Footnote 6 Where biological or chemical indicators are not appropriate, parametric monitoring devices can be used to capture cycle parameter (e.g., time, temperature, and pressure) to confirm that the conditions have been met for effective decontamination. A thermocouple is an application-specific parametric device used for the validation and verification of heat-based decontamination technologies
15.3 Chemical Disinfectants
Chemical disinfectants are generally used for the decontamination of surfaces and equipment that cannot be autoclaved, specimen and sample containers to be removed from the containment zone or biological safety cabinet (BSC), spills of infectious materials, and rooms and animal cubicles. The use of disinfectants can impact worker safety directly (e.g., direct exposure to a hazardous chemical) or indirectly (e.g., exposure to viable pathogens when an inappropriate disinfectant is selected). It is important that containment zone personnel are knowledgeable about the products required for the disinfection of the infectious material and toxins with which they will be working, including the recommended directions for use (e.g., application method, concentration, contact time, PPE, first aid, disposal) and chemical characteristics (e.g., toxicity, chemical compatibility, storage stability, active ingredient, identity, concentration).
Product effectiveness depends on the active ingredient(s) and the identity and concentration of other ingredients in the formulation. There are usually striking differences between the activities of disinfectants when used under actual laboratory conditions as opposed to the controlled, standardized testing methods (e.g., Association of Analytical Communities [AOAC International], American Society for Testing and Materials [ASTM]) used to generate efficacy data for product registration. The evaluation of disinfectant efficacy may be quantitative, semi-quantitative, or qualitative in nature. These differences are usually due to the variability in resistance to disinfection between surrogate strains used for standardized testing methods, and strains used in the laboratory. Also, environmental conditions such as temperature, relative humidity, and even water hardness are variable in laboratory settings but controlled in standardized disinfectant tests. It is therefore difficult to make generalizations about contact times and concentrations needed to kill specific pathogens. It is advisable for laboratories to conduct in-use disinfectant efficacy testing to evaluate a product's performance under their specific conditions of use.
A number of standardized tests exist to evaluate the efficacy of liquid disinfectants. Both ASTM International and AOAC International have approved disinfectant efficacy test methods. ASTM standard E2197-11, in particular, describes a basic method involving the artificial contamination of a surface (carrier disk) with test microorganisms, and subsequent exposure to the liquid disinfectant at different concentrations or for different contact times. Footnote 7 In general these tests comprise four basic steps which can be adopted for laboratory in-use disinfectant testing.
- Apply a known quantity of the microorganism in use in the laboratory to a carrier material or vessel. This quantity should be representative of the concentrations typically encountered in the laboratory.
- Apply the test disinfectant to the carrier material or vessel for the contact time used in the laboratory.
- Neutralize the disinfectant to halt its action. This can be accomplished by dilution or addition of growth media or other suitable reagent known to neutralize the active ingredients of the disinfectant.
- Assess the viability of the microorganism in a suitable growth medium.
If the microorganism survives, altering the contact time or concentration of the disinfectant, or both, may be required to achieve the desired level of disinfection. Factors that may affect the efficacy of the disinfectant are outlined in Section 15.3.1.
15.3.1 Selection of Chemical Disinfectants
The selection of an appropriate chemical disinfectant is dependent on a variety of factors, including the resistance of the infectious material or toxin, the method of application (e.g., liquid or gaseous), and the nature of the material to be disinfected (e.g., hard surface, porous materials). Organic load, concentration, contact time, temperature, relative humidity, pH, and stability can also impact the efficacy of a chemical disinfectant. Table 15-1 describes the susceptibility of pathogens to chemical disinfectants and those reported to be effective against them.
| Susceptibility | Pathogen | Disinfectants reported to be effective |
|---|---|---|
| Extremely resistant | Prions |
|
| Highly resistant | Protozoal oocysts |
|
| Bacterial endospores |
| |
| Resistant | Mycobacteria |
|
| Non-enveloped viruses |
| |
| Susceptible | Fungal spores |
|
| Gram-negative bacteria |
| |
| Gram-positive bacteria | ||
| Enveloped viruses | ||
| Highly susceptible | Mycoplasma |
|
Adapted from: Quinn, P. J., & Markey, B. K. (1991). Disinfection and Disease Prevention in Veterinary Medicine. Footnote 8
15.3.1.1 Organic Load
Organic matter (e.g., tissue, blood, bedding, feces) protects microorganisms, toxins, and prions from contact with disinfectants and can neutralize many germicides (e.g., NaOCl). Pre-cleaning with a detergent to remove bedding, litter, and feed prior to disinfection reduces organic load and achieves proper disinfection. It is important that pre-cleaning be carried out in a manner that avoids personnel exposure to infectious or potentially infectious material, and all cleaning materials are decontaminated prior to disposal. Pre-cleaning prior to disinfection may not always be appropriate and, in these cases, disinfectants that remain active in the presence of considerable amounts of organic material are a suitable alternative (e.g., phenolic disinfectants). It may be appropriate to saturate the contaminated material with a disinfectant, allowing it to remain wet for a long contact time (e.g., 30 minutes), then dispose of gross contamination and thoroughly clean surfaces before reapplying the disinfectant. Footnote 6 Footnote 8 Footnote 9
15.3.1.2 Concentration
The disinfection process is generally quicker when a higher concentration is used. High concentrations of certain chemicals may cause damage to surfaces or tissues; however, if the concentration is reduced to avoid damage, the disinfectant may no longer possess sufficient germicidal activity to be effective. It is therefore important to determine the concentration at which the disinfectant can inactivate the organism, but will not damage other materials. Footnote 6 Footnote 8 Footnote 9
15.3.1.3 Contact Time
The contact time is the period of time during which the treated surface remains saturated with the disinfectant. An effective contact time will depend on the disinfectant and the microorganisms, toxins, or prions that are present. Fast-acting disinfectants are preferable because longer contact times may be difficult to achieve. Although alcohols may have bactericidal and fungicidal activity after an extended contact time (e.g., 10 minutes), they are unlikely to remain on surfaces this long because they evaporate. Footnote 6 Footnote 8 Footnote 9
15.3.1.4 Temperature
Elevated temperatures generally enhance germicidal action; however, elevated temperatures may accelerate evaporation, thus reducing contact time. Footnote 8 Footnote 9 Lower temperatures are also a concern as the efficacy of disinfectants can be markedly reduced. This should be considered when decontaminating materials in a refrigerator, freezer, or low-temperature centrifuge.
15.3.1.5 Relative Humidity
Relative humidity can influence the activity of some disinfectants, particularly formaldehyde. The antimicrobial activity of formaldehyde gas fumigation is maximized at a relative humidity in excess of 70%. Footnote 6 Footnote 8 Footnote 9
15.3.1.6 pH
The activity of some disinfectants may be affected by pH. It is important to carefully read the directions for use and notifications regarding incompatible chemicals to ensure efficacy as well as personnel safety. Footnote 8 Footnote 9
15.3.1.7 Stability/Storage
Dilutions of some disinfectants (e.g., sodium hypochlorite [NaOCl], alkaline glutaraldehyde) may not be stable over long periods, especially in the presence of heat or light. Products should therefore be stored in a cool, dark location. Prepare only enough disinfectant for daily or weekly use (depending on shelf life). Footnote 8 Footnote 9
15.3.2 Classes of Chemical Disinfectants
Numerous types of disinfectants are available; however, the active components of disinfectants belong to relatively few classes of chemicals, and understanding the capabilities and limitations of each class will allow selection of a product based on relative effectiveness.
Table 15-2 summarizes the susceptibility of different types of microorganisms to several chemical disinfectants, including their effectiveness and the contact time required to achieve disinfection. Toxins and prions are resistant to many chemical disinfectants; the specific considerations for decontamination of toxins and prions are discussed in Sections 15.11 and 15.12, respectively. Table 15-3 describes the disadvantages of the same chemical disinfectants.
| Chemical Disinfectant | Commonly Available Form | Effective Against | Contact Time | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Bacteria | Viruses | Fungi | |||||||
| Vegetative | Mycobacteria | Spores | Enveloped | Non-enveloped | Fungi | Fungal Spores | |||
| Chlorine | Liquid, powder and tablet | + | + | + | + | + | + | + | Generally short; longer for bacterial spores (≥ 30 min) |
| Iodine | Aqueous solutions, tinctures and iodophors | + | L | L | + | L | + | L | Generally short for vegetative bacteria and enveloped viruses; contact time for other organisms is product-specific |
| Alcohol | Ethyl or isopropyl alcohol; 70% in water is most effective | + | + | - | + | L | + | L | Generally short for vegetative bacteria and enveloped viruses; longer for fungi and mycobacteria |
| Phenolics | Wide variety; generally used as substituted phenols in combination with detergents | + | V | - | + | - | V | V | |
| Quaternary ammonium compounds | Wide variety available with built-in detergent action | + | - | - | + | - | + | - | |
| Glutaraldehyde | 2% acidic solution supplied with a bicarbonate compound | + | + | + | + | + | + | + | ≥ 20 min required for non-enveloped viruses and mycobacteria; >3 hours required for bacterial spores |
| Formaldehyde | Available as solid paraformalde-hyde and liquid formalin | + | + | + | + | + | + | + | |
| Hydrogen peroxide (H2O2) | Accelerated formulations and 30% solutions in water | + | + | + | + | + | + | + | Short contact time with 6% H2O2, for all viruses, vegetative bacteria, fungi, mycobacteria, and some bacterial spores; higher concentrations and longer contact times required for sporicidal activity |
| Chlorhexidine | 4% solution of chlorhexidine gluconate in a detergent base and concentrated alcohol-based solutions | +/L * | - | - | + | - | L | - | |
| +: effective; L: limited activity; V: variable activity; -: no activity | |||||||||
| Chemical Disinfectant | Disadvantages |
|---|---|
| Chlorine |
|
| Iodine |
|
| Alcohol |
|
| Phenolics |
|
| Quaternary ammonium compounds |
|
| Glutaraldehyde |
|
| Formaldehyde |
|
| Hydrogen peroxide |
|
| Chlorhexidine |
|
15.4 Autoclaves
Infectious material and toxins, together with associated waste (e.g., petri dishes, pipettes, culture tubes, and glassware), can be effectively decontaminated in either a gravity displacement autoclave or a pre-vacuum autoclave. Gravity displacement autoclaves allow air to escape through the bottom of the chamber as steam displaces it from above. In order for this system to function efficiently, care should be taken to ensure that the valves remain unobstructed and that the chamber is not overfilled. Pre-vacuum autoclaves remove air from the chamber by employing a vacuum before letting saturated steam enter the autoclave chamber (except during liquid cycles). Pre-vacuum autoclaves resolve the air entrapment problems that are often encountered in gravity displacement autoclaves.
Autoclaves can be designed with a single door or with double doors. Double-door autoclaves are installed on the containment barrier, typically in high containment zones, to facilitate the decontamination and movement of waste and other contaminated material out of the containment zone. The effectiveness of decontamination by steam autoclaving is dependent on the temperature to which the material is subjected as well as the length of time it is exposed. Proper operation, loading, and monitoring of autoclaves are critical to ensure decontamination is achieved. Particular attention should be given to packaging, including the size of containers and their distribution in the autoclave so that the innermost regions of bags and containers reach and maintain the temperature required for sterilization. Arranging items in a manner that allows the free circulation and penetration of steam will help achieve effective decontamination of waste.
15.4.1 Recommended Procedures for the Use of Autoclaves
Some points to consider or to include when developing the standard operating procedures (SOPs) for the safe and effective use of a specific autoclave within the containment zone are provided in the following sections.
15.4.1.1 Before Loading the Autoclave
- Before opening the door of a double-door barrier autoclave, confirm that the door on the opposite side of the autoclave is closed (i.e., through visual and audible alarms).
- Check inside the autoclave for any items left by the previous user that could pose a hazard (e.g., sharps).
- Clean the drain strainer.
- Confirm that any plastic materials used, including bags, containers and trays, are compatible with autoclaving. Some bags can impede steam penetration while others may melt during the cycle.
- Avoid overloading containers and bags (they should never be more than 3/4 full).
- Autoclave bags should be closed loosely to allow adequate steam penetration.
- Loosen the caps of liquid containers to prevent bottles from shattering during pressurization. This should be done immediately prior to loading in order to minimize the risk of exposure or contamination if the container is tipped. Vented caps may be a suitable alternative.
15.4.1.2 Loading the Autoclave
- Load autoclave according to the manufacturer's recommendations.
- Arrange containers, bags, and trays in a manner that allows steam to circulate freely around all items. Avoid stacking or crowding items.
- Consider placing containers and bags in trays with a solid bottom and walls to contain spills.
- Avoid placing individual containers on the floor of the autoclave.
- Make sure the door of the autoclave is fully closed (i.e., latched) and that the correct cycle has been selected.
15.4.1.3 Unloading the Autoclave
- Verify the autoclave cycle log to ensure decontamination parameters have been achieved.
- Visually check the pressure gauge to ensure that the pressure has decreased inside the chamber.
- Don PPE, including eye protection, heat-resistant long-cuff gloves, rubber apron, rubber sleeve protectors, and, when handling sharps, cut-resistant gloves.
- Materials removed from the autoclave after effective (i.e., verified) decontamination should be placed in disposal bags that clearly indicate that the waste has been decontaminated, and any biohazard symbols removed or defaced.
15.4.1.4 Verifying the Autoclave Run
- After decontaminated material has been removed from the autoclave, and prior to disposal, it is important to verify that the run has been effective (i.e., that all validated parameters have been reached). Parametric monitoring devices, chemical indicators and integrators, and biological indicators can be used for routine monitoring of the decontamination process.
- Remove the indicator or integrator from the autoclaved material and visually inspect. Chemical indicators and integrators provide immediate information on the parameters to which they react. If it was required to also include biological indicator, the material cannot be released for disposal or reuse until the results of the biological indicator are known.
- Biological indicators require incubation for a pre-determined period of time before reading.
15.4.2 Recommended Procedures for Efficacy Monitoring of Autoclaves
Recommended procedures for efficacy monitoring (i.e., verification) of autoclave cycles at 121°C are described below, and should be performed at a frequency determined through an LRA, taking into consideration the frequency of use of the autoclave. For extended autoclave cycles up to 134°C, chemical integrators or independent temperature monitoring devices (e.g., thermocouples) may be used for verification.
- Place biological indicators (e.g., ampoules containing 104-106 colony forming units [cfu] of Geobacillus stearothermophilus spores) in the centre of the load (i.e., the most difficult areas of the load to decontaminate). Different load types (e.g., reusable PPE, solid waste, liquid waste) should be tested separately.
- Leave a positive control biological indicator of the same lot number outside of the autoclave.
- Process the load according to the applicable SOPs, taking into account the lag time required for the temperature at the centre of the load to reach the sterilization temperature; this time will vary depending on the nature of the waste to be sterilized. For example, G. stearothermophilus spores exposed to 121°C are killed in 15 minutes; however, the total cycle time and the temperature required will depend on the contents of the load.
- Retrieve the biological indicators after completion of the cycle.
- Do not dispose of waste until it has been confirmed that it has been effectively decontaminated (i.e., absence of growth in the autoclaved biological indicators).
- Incubate the biological indicators, including the positive control, for the appropriate amount of time and examine for growth. Growth in the autoclaved biological indicators indicates sterilization failure. The absence of growth indicates that sterilization, and hence a reduction equivalent to the initial concentration in the positive control of G. stearothermophilus spores, was achieved. Rapid readout biological indicators containing G. stearothermophilus spores can also be used. After incubating for 1-3 hours at 56°C in a fluorometer, the illumination of a red light indicates fluorescence and sterilization failure, whereas the illumination of a green light indicates non-fluorescence and successful sterilization.
- Failure to achieve sterilization may be due to insufficient sterilization time, pressure, or temperature due to user error (e.g., use of wrong cycle program), instrument failure, improper loading, or overloading of the autoclave (i.e., the centre of the load failed to reach and maintain the temperature required for sterilization). Should this occur, the failure should be investigated and corrected. If the cause is found to be instrument failure, the autoclave will have to be repaired and the process re-validated before repeating. Once corrected, the waste is re-autoclaved (i.e., the autoclave process is repeated) prior to disposal.
15.5 Gaseous Decontamination
Gaseous decontamination is generally used in high containment zones under particular circumstances (e.g., after a spill or accidental release of infectious material or toxins, before the removal of large equipment, before maintenance work on contaminated systems, before retesting heating, ventilation, and air conditioning [HVAC] control systems). Gaseous decontamination of rooms usually requires the use of hazardous chemicals (e.g., formaldehyde, vaporized hydrogen peroxide [VHP], chlorine dioxide [ClO2], ethylene oxide). For this reason, it is important that gaseous decontamination be performed by personnel who have been trained in the procedure and the use of appropriate PPE, including respiratory protection. The two-person rule (also commonly known as a "buddy system"), where two authorized and trained individuals are present at all times, applies to this procedure. It is recommended that, prior to gaseous decontamination, the room or laboratory is leak tested with a tracer gas, such as mint, in order to identify and mitigate leaks.
Formaldehyde gas is a colourless, corrosive, flammable gas that acts as an alkylating agent, binding to specific sites on proteins, RNA, or DNA; it is generated by the depolymerization of paraformaldehyde, and in the presence of water vapour used effectively as a decontaminant. Footnote 15 The typical protocol for decontamination using this bactericide involves a 12 hour exposure (6 hour exposure for BSCs) at a relative humidity of 60-90% and a temperature between 15°C and 32°C. This ensures a survival rate of less than one bacterial spore in a million for bacterial spores known to be most resistant to formaldehyde gas. Formaldehyde gas can be neutralized by ammonia gas, which is generated by the thermal decomposition of ammonium bicarbonate or ammonium carbonate.
VHP is an oxidizing agent that is effective against many different types of pathogens, including bacterial spores. It has been proposed as a safer alternative to gaseous decontamination with formaldehyde. Footnote 2 This decontamination method does not generate harmful by-products since VHP is broken down into non-toxic oxygen and water. VHP is compatible with a broad range of materials and finishes; however, it has been shown to be incompatible with some materials such as natural rubbers and some plastics and paints. Recent advances in VHP technology have permitted the decontamination of increasingly larger spaces, from small pass-through chambers to areas up to 280 m3 (10, 000 cubic feet) and beyond.
Dry fog is not gaseous, but rather consists of ultrafine droplets of peracetic acid and hydrogen peroxide (H2O2). It is a powerful oxidizer that breaks down organic components of microorganisms, destroying their structure. As with VHP, dry fog also breaks down to harmless components and leaves no residue. It is considered compatible with most materials, including electronics; however, as the fog consists of particles (about 7.5 µm), it does not penetrate materials and is not effective for the decontamination of high efficiency particulate air (HEPA) filters.
ClO2 is a selective oxidant that reacts primarily with organic compounds that are highly reduced (e.g., alcohols, aldehydes, ketones, tertiary amines, and sulphur-containing amino acids). ClO2 displays broad-spectrum bactericidal, fungicidal, and virucidal activity, and is effective against bacterial spores. Footnote 16 Unlike vapours, ClO2 is a true gas at standard room temperatures and is, therefore, not affected by temperature gradients that can cause condensation and concentration inconsistencies. ClO2 demonstrates superior distribution compared to VHP, and as a selective oxidant, it is compatible with many standard materials, including paper, plastic, stainless steel, polyvinyl chloride (PVC), anodized aluminum, and wood. Footnote 16Footnote 17
Pre-cleaning of all surfaces to remove superficial organic matter and dirt prior to performing gaseous decontamination allows the gas to effectively contact all surfaces. H2O2 and dry fog decontamination in particular require a clean surface since they do not have any penetrating power. Placing biological indicators in various locations including areas difficult for gas to reach or penetrate (e.g., corners, drawers, crevices) provides a means to evaluate the effectiveness of the gaseous decontamination process. Chemical indicators can be used in conjunction with biological indicators to provide an immediate confirmation that the gas has reached all areas targeted, but the area is not considered decontaminated until the results of the biological indicators are known. Geobacillus stearothermophilus is the preferred biological indicator organism for testing the efficacy of formaldehyde, VHP, and ClO2. The target value for decontamination within the room spaces and BSCs is a 6-log10 (i.e., 99.9999%) reduction of viable spores. Footnote 18 Footnote 19 Footnote 20
15.6 Effluent Decontamination Systems
Liquid waste treatment systems are designed to prevent the release of untreated materials into sanitary sewers, and ultimately, the environment. The requirements relating to effluent decontamination systems are listed in Matrix 3.8 of the CBS. Effluent decontamination systems may also be a design consideration for other containment zones depending on the activities planned and the pathogens being handled (e.g., large scale production areas). Where an effluent decontamination system is present, it usually serves as the primary decontamination technology to treat all liquid waste from sources within or serving the containment zone, including sinks, showers, toilets, autoclaves, washing machines, and floor drains. In some areas, liquid waste may be treated with a validated decontamination process or procedure prior to disposal to the effluent decontamination system; in this case, the effluent decontamination system acts as a secondary decontamination technology (i.e., backup system). Effluent decontamination systems are commonly heat-based; however, a chemical-based system may be practical on a smaller scale where small volumes of liquid effluent require treatment.
In traditional effluent decontamination systems, liquid waste is collected in a large tank. When the tank is full, the liquid is heated or chemically treated and, after allowing sufficient time to complete the decontamination, the tank is drained into the sanitary sewer. A uniform temperature or chemical concentration in a large tank can be a challenge to achieve, which can lead to inadequate decontamination. To mitigate this risk, some systems include features to help achieve and maintain a uniform temperature, such as paddles to ensure constant mixing of the effluent, or steam jackets that surround the effluent vessel shell. Continuous effluent decontamination systems have also been recently introduced. In this type of system, the effluent is collected in a large tank and continuously streamed through a retention pipe where the decontamination process takes place. As it flows through the retention pipe at a pre-determined rate, the effluent is treated to a specific parameter (e.g., heat, chemical) for a specific period of time to achieve effective decontamination.
The decontamination parameters (e.g., time, temperature, chemical concentration) of the effluent decontamination system are validated to confirm they will be effective against the infectious material or toxins of concern. Parameters such as internal temperature and pressure of the effluent and the decontamination time are recorded throughout the cycle to evaluate the effectiveness of the process. Alarms are connected to the system to permit the timely detection of a failure. A "fail-safe" configuration will help to prevent untreated waste from leaving the system in the event of a malfunction. Performance verification of effluent decontamination systems should include a brief description of the run criteria for the specific pathogen(s) in use, the procedures for microbiological challenge and verification, trending charts, digital printouts, and other data, as necessary.
The quality of the treated liquid waste released from the effluent decontamination system has to meet the standards specified in applicable environmental regulations and bylaws (provisions related to temperature, chemical and metal content, suspended solids, oil and grease, and biochemical oxygen demand). For example, when chemical residues (e.g., chlorine and ozone) are not neutralized prior to release, they can generate noxious fumes and waterborne residues or by-products (e.g., bromine in salt water). This can be harmful to aquatic animals, and to humans if inhaled, absorbed, or ingested. With other types of treatment, such as heat, post-treatment cooling of the decontaminated waste may be required before discharge into municipal drains or waterways.
Although not specifically aimed at containment zone waste decontamination, the principles and physical/chemical processes described in the Manual of Diagnostic Tests for Aquatic Animals published by the World Organisation for Animal Health (OIE; Office International des Épizooties) may be applicable to the design of a waste treatment system. Footnote 21 Please refer to the OIE website for more information (http://www.oie.int).
15.7 Irradiation
Gamma irradiation (e.g., Cobalt-60) can be used for the decontamination of heat-sensitive materials and is effective at decontaminating the chemicals and solvents that may be used in higher containment zones; however, it may not be capable of effectively decontaminating certain pathogens (e.g., bacterial spores). Footnote 22Footnote 23 The efficacy of this process is dependent on the penetration of the materials by gamma irradiation, which is a function of the density of the treated substance and the strength of the irradiation source.
Microwave irradiation is not widely used as a means of decontamination in containment zones. Similar to autoclaving, this process is based on the use of heat to eliminate viable microorganisms and, for this reason, autoclaving is usually the technology of choice. The efficacy of microwave irradiation is dependent on the wavelength of the irradiation, the duration of exposure, and the moisture content of the material to be decontaminated.
Ultraviolet (UV) irradiation should never be used as the sole means of decontamination in containment zones. Since UV irradiation lacks penetrating capacity, it is only effective in reducing airborne and surface contamination. If UV irradiation is being used in conjunction with other decontamination processes, UV lights should be maintained (e.g., properly cleaned) and periodically verified for function (e.g., emitting appropriate intensity of light).
Validation methods for irradiation sterilization require the use of biological indicators such as Bacillus pumilus spore strips. Footnote 24 Several spore strips should be distributed throughout the sample being sterilized or the sterilization chamber, depending on the type of validation. A positive control (i.e., unprocessed spore strip) should be used along with the testing strips.
15.8 Incineration
Effective incineration depends on proper equipment design, time, temperature, turbulence, and air required for complete oxidation, as well as careful loading of the unit. Incinerators with a single combustion chamber are generally not effective for the disposal of animal carcasses and plastics since these materials may not be completely destroyed. Footnote 25 Modern incinerators that have two chambers, with an ideal temperature of at least 800°C in the primary chamber and at least 1000°C in the secondary chamber, may be effective. Loads with high moisture content may lower the processing temperature, and sawdust may be added to enhance stability. Footnote 26 There are no microbial standards for stack emissions, but there are for emission of particulate matter and selected chemical contaminants. Footnote 27 Provincial or territorial regulatory authorities should be consulted for additional requirements related to incinerator operations and emissions.
Autoclaving is the preferred method to decontaminate materials, equipment, and waste at the containment barrier prior to its removal from high containment zones for transporting to an incinerator. Material to be incinerated should be packaged in leak-proof plastic bags, even if previously decontaminated. Off-site transportation of this material is subject to provincial or territorial legislation. It is important that written protocols for the packaging, labelling, storage, and transportation of waste materials destined for the incinerator be developed and followed by all personnel.
Effective waste management training for containment zone personnel will ensure that they are aware of the types of materials that may be incinerated. The effective operation of the incinerator is highly dependent on the material and the volume of material that is being incinerated. Additional training for personnel responsible for loading, operating, and cleaning the incinerator is needed to ensure that protocols are understood and adhered to, and PPE, such as a respirator for cleaning out ashes and a harness for loading, is used properly. Generally, ash generated by incinerators can be handled as normal waste.
15.9 Dunk Tanks
Dunk tanks are located on the containment barrier to allow for the safe removal of material and samples from the containment zone via surface decontamination. It is critical to use a disinfectant that is effective against the infectious material or toxins in use, and to use the appropriate concentration of disinfectant with sufficient contact time to effectively surface decontaminate vessels that are immersed in the dunk tank. The dunk tank should be inspected regularly as many disinfectants can be corrosive and degrade the dunk tank surfaces. Using a dunk tank with a lining that protects against corrosion may be preferable.
It is important that an adequate volume of disinfectant is maintained in the tank; regular inspections of the disinfectant level will help to identity when additional disinfectant is needed. Visual or audible alarms included on dunk tanks to signal low level of disinfectant require routine testing to verify that they function as intended (CBS Matrix 5.1). Disinfectants have varying shelf lives and the dunk tank solution is to be replaced or replenished as necessary in order to maintain the required disinfectant concentration.
15.10 Animal Carcasses and Anatomical Waste
Animal waste, discarded surgery and necropsy tissues, and whole carcasses can be decontaminated by heat or chemical means. In general, specimens and tissues can be autoclaved effectively, as described in Section 15.4 of this chapter. Whole infected carcasses may require rendering at high temperature, incineration, or chemical decontamination (e.g., alkaline hydrolysis). A modified rendering process has been shown to be an effective alternative and has been successfully used to decontaminate infected animal carcasses. Footnote 28
Alkaline hydrolysis is a process by which animal carcasses and tissues are subjected to a strong alkali, high temperature, and high pressure. A tissue digester would be one example of a decontamination technology that relies on an alkaline hydrolysis process. In general, this method involves a temperature of 150°C and a pressure of 483 kPa (70 PSI), with a total process time of 3-8 hours; however, the exact temperature and time required to achieve effective decontamination is dependent on factors such as the pathogen of concern, and the size/amount of the carcass/tissue to be decontaminated. Footnote 29 The final products of this process are amino acids, peptides, sugars, nutrients, soap, bones and teeth.
Rendering is a process by which animal carcasses and tissues are subjected to high temperature and pressure. The pressure vessel, typically designed with a shaft and paddles, uses steam to sterilize and render the waste non-infectious. When properly processed, the final product is somewhat dry and can be sent for disposal (i.e., to landfill). Rendering is most often used for larger animal carcasses and tissues.
Composting is a naturally occurring process that involves the aerobic decomposition of tissue by bacteria and fungi, and may be used to dispose of animal carcasses and anatomical waste. When proper techniques are employed, composting is a well-established pathogen reduction technology that has demonstrated the ability to reduce nearly all pathogenic viruses, bacteria, fungi, protozoa (including cysts), and helminths ova to acceptably low levels. Footnote 30 With the exceptions of endospore-forming bacteria (e.g., Bacillus cereus) and prions, composting of waste from CL2 LA zones (i.e., CL2-Ag) and from animals in confinement may be an acceptable method to decontaminate waste, provided that the method was appropriately validated. Composting procedures should be developed and followed in accordance with applicable provincial, territorial, and municipal legislation, and may not be an option in all jurisdictions.
15.11 Thermal and Chemical Decontamination of Biological Toxins
Given the wide variety of biological toxins and the considerable differences in their physical properties, it is impossible to provide a standardized set of thermal or chemical decontamination parameters that apply to all circumstances. It is the responsibility of the facility where the toxins are handled or stored to ascertain the risks and determine how best to mitigate them, including appropriate and effective inactivation methods.
In an effort to provide a general recommendation for toxin decontamination, more stringent times, temperatures, and concentrations have been outlined below that are considered to be effective against most toxins; however, exceptions to these recommendations do exist and are discussed accordingly.
15.11.1 Thermal Decontamination
Moist-heat (i.e., autoclaving) methods of inactivation with temperatures in excess of 121°C for 60 minutes will permit adequate inactivation of most biological toxins, including proteinaceous bacterial toxins; however, this approach is not suitable for the inactivation of low-molecular-weight, heat-stable toxins, including anthrax toxins, perfringolysin O, and mycotoxins. Footnote 31 Dry-heat methods (e.g., incineration), at sustained temperatures of at least 815°C for 10 minutes, are effective for the inactivation of most biological toxins. Footnote 31Footnote 32 For heat-stable toxins, effective chemical decontamination methods should be applied.
15.11.2 Chemical Decontamination
A solution containing 2.5% NaOCl and 0.25N NaOH with a contact time of at least 30 minutes will permit adequate inactivation of most biological toxins, including peptide toxins and mycotoxins. Footnote 32 In addition, select toxins are susceptible to other chemicals such as formaldehyde, glutaraldehyde, and ethanol.
15.11.3 Decontamination Parameters
Examples of thermal or chemical inactivation methods for certain toxins are provided below:
- Toxins A and B (Clostridium difficile) are susceptible to treatment with 2% glutaraldehyde. Footnote 33
- Listeriolysin O (Listeria monocytogenes) is inactivated by heating at 80°C for 3 minutes. Footnote 34
- Pasteurella multocida toxins are inactivated by heating at 56°C for 30 minutes. Footnote 35
- Items grossly contaminated with mycotoxins require treatment with a solution containing 2.5% NaOCl and 0.25N NaOH for 2-8 hours. Footnote 31Footnote 32
- The treatment of aflatoxin B1 (derived from Aspergillus flavus and Aspergillus parasiticus) with NaOCl can lead to the formation of a potent carcinogen and mutagen. To prevent this, the treatment solution should be diluted such that the final concentration of NaOCl is between 1 and 5%, followed by the addition of acetone to a final concentration of 5% (vol/vol). Footnote 31Footnote 32Footnote 36
15.12 Additional Considerations for Prion Decontamination
Prions are resistant to normal decontamination procedures and processes, including moist heat from conventional autoclaving, irradiation, and chemical inactivation (e.g., formalin, alcohols). These processes can marginally reduce infectivity, but few (e.g., incineration, repeated alkali autoclaving at pH 13) are highly effective at eliminating infectious prions. Footnote 37 Combinations of physical and chemical treatment processes are recommended to decontaminate equipment, reusable materials, and waste products that are not suitable for incineration, as they can achieve greater prion inactivation efficacy than treatment with chemical agents alone. Autoclaves can be used as a single-step or a two-step method of decontamination for containment zones where prions are handled. Autoclaves used as part of a two-step process (i.e., at 121°C) can be validated with biological indicators, while those used as part of a single-step process (i.e., 134°C) are validated using thermocouples/temperature probes to demonstrate that they are operating as specified. The following are considerations for the decontamination of prions:
- For animal carcasses, tissue waste, bedding, excrement, and some disposable laboratory materials: Incineration at 850°C or alkaline hydrolysis using a pressurized vessel at 150°C are acceptable treatment methods that have demonstrated complete inactivation of prions. Footnote 5Footnote 38 In general, incineration at 850°C is the recommended method to achieve effective decontamination of all prion disease agents. Footnote 39
- For reusable instruments that are not heat-sensitive: Autoclaving at 134°C for 1 hour (i.e., single-step decontamination process) or a chemical treatment with 1N NaOH or NaOCl followed by autoclaving at 121°C for 1 hour (i.e., two-step process) is acceptable for prion decontamination. Footnote 5Footnote 9
- For heat-sensitive reusable instruments and surfaces: 2N NaOH or 2% available chlorine, with a contact time of 1 hour at 20oC, is effective. Footnote 5Footnote 26Footnote 40
- For wastewater: Decontamination of liquid effluent at 134°C for 1 hour is the indicated method of decontamination for prion decontamination. Effluent decontamination systems should be designed to treat liquid waste at 134°C for 1 hour. Footnote 26 Where the animals necropsied are not known to be infected with prions (e.g., veterinary diagnostic facilities), an effluent decontamination system is not required in post mortem rooms (PM rooms). However, in the event that a positive animal is detected, operational procedures should be in place to collect and treat liquid waste. For example, plastic or absorbent pads may be used to contain liquids during necropsy of animals that exhibit signs of neurological disease.
- For HEPA filters: In a BSC, bag-in/bag-out filters are recommended because formaldehyde fumigation is ineffective against prions. It has been demonstrated that VHP provides a significant reduction in infectivity; however, it is necessary to evaluate the efficacy of VHP decontamination, including establishment and validation of VHP concentration and exposure time, prior to implementing VHP as a decontamination method. Footnote 41Footnote 42 Decontamination of filters with VHP followed by incineration is considered to be an acceptable option for safe removal and disposal of filters.
There are additional precautions that should be considered when autoclaving chemically treated (e.g., NaOH, NaOCl) waste. This material can be damaging to equipment; therefore, proper containers should be used. In addition, personnel should be cautious when handling hot NaOH (post autoclave) in order to prevent potential exposure to gaseous NaOH.
References
- Footnote 1
- Government of Canada. (2015). Canadian Biosafety Standard (2nd ed.). Ottawa, ON, Canada: Government of Canada.
- Footnote 2
- Favero, M. S., & Arduino, M. J. (2006). Decontamination and Disinfection. In Fleming, D. O., & Hunt, D. L. (Eds.), Biological Safety: Principles and Practices (4th ed., pp. 373-381). Washington, DC, USA: ASM Press.
- Footnote 3
- Weber, A. M., Boudreau, U. V., & Mortimer, V. D. (2000). A Tuberculosis Outbreak Among Medical Waste Workers. Journal of the American Biological Safety Association. 2:70-88.
- Footnote 4
- Collins, C. H., & Kennedy, D. A. (1999). Laboratory- Acquired Infections. In Laboratory- Acquired Infections: History, Incidence, Causes and Preventions (4th ed., pp. 1-97). Oxford, UK: Butterworth-Heinemann.
- Footnote 5
- United States Department of Health and Human Services, United States Centers for Disease Control and Prevention, & United States National Institutes of Health. (2009). Biosafety in Microbiological and Biomedical Laboratories (5th ed.). Washington, DC, USA: United States Government Printing Office.
- Footnote 6
- Rutala, W. A., Weber, D. J., & Healthcare Infection Control Practices Advisory Committee. (2008). Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. Washington, DC, USA: United States Government Printing Office / United States Centers for Disease Control and Prevention.
- Footnote 7
- ASTM E2197-11, Standard Quantitative Disk Carrier Test Method for Determining Bactericidal, Virucidal, Fungicidal, Mycobactericidal and Sporicidal Activities of Liquid Chemical Germicides. (2011). West Conshohocken, PA, USA: American Society for Testing and Materials.
- Footnote 8
- Block, S. S. (Ed.). (2001). Disinfection, Sterilization, and Preservation (5th ed.). Philadelphia, PA, USA: Lea & Febiger.
- Footnote 9
- McDonnell, G. (2007). Antisepsis, Disinfection, and Sterilization. Washington, DC, USA: ASM Press.
- Footnote 10
- British Columbia Centre for Disease Control. (2003). A Guide to Selection and Use of Disinfectants. Retrieved 11/03, 2015 from http://www.bccdc.ca/NR/rdonlyres/EAA94ACF-02A9-4CF0-BE47-3F5817A25669/0/InfectionControl_GF_DisinfectntSelectnGuidelines_nov0503.pdf
- Footnote 11
- Johnson, C. H., Marshall, M. M., DeMaria, L. A., Moffet, J. M., & Korich, D. G. (2003). Chlorine Inactivation of Spores of Encephalitozoon spp. Journal of Applied and Environmental Microbiology. 69:1325-1326.
- Footnote 12
- Kennedy, J., Bek, J., & Griffin, D. (2000). G1410 Selection and Use of Disinfectants. Historical materials from University of Nebraska. USA: University of Nebraska-Lincoln Extension, Institute of Agriculture and Natural Resources. Paper 105. Retrieved 11/03, 2015 from http://digitalcommons.unl.edu/extensionhist/105.
- Footnote 13
- Russel, A. D. (1986). Chlorhexidine: Antibacterial Action and Bacterial Resistance. Journal of Infection. 14:212-215.
- Footnote 14
- Favero, M. S. (1998). Developing Indicators for Monitoring Sterilization. In Rutala, W. A. (Ed.), Disinfection, Sterilization, and Antisepsis in Healthcare (pp. 119-132). Washington, DC, USA: Association for Professionals in Infection Control and Epidemiology, Inc.
- Footnote 15
- Luftman, H. S. (2005). Neutralization of Formaldehyde Gas by Ammonium Bicarbonate and Ammonium Carbonate. Applied Biosafety: Journal of the American Biological Safety Association. 10(2):101-106.
- Footnote 16
- Czarneski, M. A., & Lorcheim, P. (2005). Isolator Decontamination Using Chlorine Dioxide Gas. Pharmaceutical Technology. 124-133.
- Footnote 17
- Luftman, H. S., Regits, M. A., Lorcheim, P., Czarneski, M. A., Boyle, T., Aceto, H., Dallap, B., et al. (2006). Chlorine Dioxide Gas Decontamination of Large Animal Hospital Intensive and Neonatal Care Units. Applied Biosafety: Journal of the American Biological Safety Association. 11(3):144-154.
- Footnote 18
- National Standards Foundation (NSF). (2014). NSF/ANSI 49-2014 Annex K - Protocol for the Validation of Alternate Biosafety Cabinet Decontaminating Methods and Agents. Ann Arbour, MI, USA: National Sanitation Foundation / American National Standards Institute.
- Footnote 19
- Lewis, C., Batdorf, N., Klinedinst, K., Dabisch, P., & Pitt, L. (2011) . Efficacy of Vaporous Hydrogen Peroxide Against Bacillus atrophaeus and Bacillus anthracis Spores. Fort Detrick, MD, USA: Center for Aerobiological Sciences, United States Army Medical Research Institute of Infectious Diseases.
- Footnote 20
- Luftman, H. S., & Regits, M. A. (2008). B. Atrophaeus and G. Stearothermophilus Biological Indicators for Chlorine Dioxide Gas Decontamination. Applied Biosafety. 13(3):143-157.
- Footnote 21
- World Organisation for Animal Health. (2014). Manual of Diagnostic Tests for Aquatic Animals. Paris, France: World Organisation for Animal Health.
- Footnote 22
- Hoile, R., Banos, C., Colella, M., Walsh, S. J., & Roux, C. (2010). Gamma Irradiation as a Biological Decontaminant and its Effect on Common Fingermark Detection Techniques and DNA Profiling. Journal of Forensic Sciences. 55(1):171-177.
- Footnote 23
- Reardon, S. (2015). US military accidentally ships live anthrax to labs. Nature. doi:10.1038/nature.2015.17653
- Footnote 24
- World Health Organization. (2013). Methods of Analysis: 5. Pharmaceutical technical procedures: 5.8 Methods of sterilization in The International Pharmacopoeia (4th ed.). Retrieved 11/03, 2015 from http://apps.who.int/phint/en/p/docf
- Footnote 25
- World Health Organization. (2004). Laboratory Biosafety Manual (3rd ed.). Geneva, Switzerland: World Health Organization.
- Footnote 26
- World Health Organization. (2000). WHO Infection Control Guidelines for Transmissible Spongiform Encephalopathies. Geneva, Switzerland: World Health Organization.
- Footnote 27
- Canadian Council of the Ministers of the Environment. (1992). Guidelines for the Management of Biomedical Waste in Canada. Mississauga, ON, Canada: Canadian Standards Association.
- Footnote 28
- Thompson, L., Best, M., & Langevin, P. (1998). Biological Efficacy Testing of Liquid Effluent and Tissue/Carcass Sterilization Systems. Mundelein, IL, USA: American Biological Safety Association.
- Footnote 29
- National Agricultural Biosecurity Center Consortium, Carcass Disposal Working Group. (2004). Chapter 6: Alkaline Hydrolysis. In Carcass Disposal: A Comprehensive Review. Retrieved 11/03, 2015 from http://krex.k-state.edu/dspace/handle/2097/662
- Footnote 30
- Wilkinson, K. G. (2007). The Biosecurity of On-Farm Mortality Composting. Journal of Applied Microbiology. 102:609-618.
- Footnote 31
- Kozlovac, J. P., & Hawley, R. J. (2006). Biological Toxins: Safety and Science. In Fleming, D. O., & Hunt, D. L. (Eds.), Biological Safety: Principles and Practices (4th ed., pp. 253-270). Washington, DC, USA: ASM Press.
- Footnote 32
- Wannemacher, R. W., & Wiener, S. L. (1997). Trichothecene Mycotoxins. In Zajtchuk, R., & Bellamy, F. F. (Eds.), Medical Aspects of Chemical and Biological Warfare. (pp. 655-676). Washington, DC, USA: Bordem Institute.
- Footnote 33
- Rutala, W. A. (1996). APIC Guideline for Selection and Use of Disinfectants. American Journal of Infection Control. 24:313-342.
- Footnote 34
- Kim, K., Murano, E. A., & Olson, D. G. (1993). Development of an Enzyme-Linked Immunosorbent assay (ELISA) for Analysis of Listeriolysin O Produced by Listeria Monocytogenes. Journal of Rapid Methods & Automation in Microbiology. 2(3):189-201.
- Footnote 35
- Rutter, J. M. (1983). Virulence of Pasteurella multocida in atrophic rhinitis of gnotobiotic pigs infected with Bordetella bronchiseptica. Research in Veterinary Science. 34:287-95.
- Footnote 36
- Castegnaro, M., Friesen, M., Michelon, J., & Walker, A. (1981). Problems Related to the Use of Sodium Hypochlorite in the Detoxification of Aflatoxin B1. American Industrial Hygienists Association Journal. 42:398-401.
- Footnote 37
- United Kingdom Department of Health, Engineering and Science Advisory Committee into the Decontamination of Surgical Instruments Including Prion Removal (ESAC-Pr). (2008). New Technologies Working Group Report on Prion Inactivating Agents. London, UK: Department of Health.
- Footnote 38
- United Kingdom Department of Health, Advisory Committee on Dangerous Pathogens, & the Spongiform Encephalopathy Advisory Committee. (2009). Transmissible Spongiform Encephalopathy Agents: Safe Working and the Prevention of Infection - Annex C: General Principles of Decontamination and Waste. Guidance from the Advisory Committee on Dangerous Pathogens and the Spongiform Encephalopathy Advisory Committee. London, UK: Department of Health.
- Footnote 39
- Leunda-Casi A., Pauwels K., Herman Ph., Verheust C., Zorzi W., Thellin O., Roels S., Van Vaerenbergh B. (2009). Risk assessment of laboratories involving the manipulation of unconventional agents causing TSE. Retrieved 11/03, 2015 from http://www.biosafety.be/CU/PDF/Report_Prions_IPH_D_2009_2505_49.pdf
- Footnote 40
- World Organisation for Animal Health. (2015). Chapter 2.4.6. Bovine Spongiform Encephalopathy. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Paris, France: World Organisation for Animal Health. Retrieved 11/03, 2015 from http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.04.06_BSE.pdf
- Footnote 41
- Fichet, G., Comoy, E., Duvall, C., Antloga, K., Dehen, C., Charbonnier, A., McDonnell, G., et al. (2004). Novel Methods for Disinfection of Prion-Contaminated Medical Devices. The Lancet. 364:521-526.
- Footnote 42
- Fichet, G., Antloga, K., Comoy, E., Deslys, J. P., & McDonnell, G. (2007). Prion Inactivation Using a New Gaseous Hydrogen Peroxide Sterilisation Process. Journal of Hospital Infection. 67:278-286.
Case Problem Answers Law for Business 19th Edition Chapter 15
Source: https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/handbook-second-edition/chapter-11-15.html